| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:29:23 UTC |
|---|
| Update Date | 2020-05-11 18:23:19 UTC |
|---|
| BMDB ID | BMDB0000365 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Epiandrosterone |
|---|
| Description | Epiandrosterone belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. Based on a literature review a significant number of articles have been published on Epiandrosterone. |
|---|
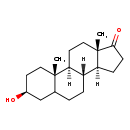
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Epiandrosterone | HMDB | | 3b-Androsterone | HMDB | | 3b-Hydroxy-17-oxo-5a-androstane | HMDB | | 3b-Hydroxy-5a-androstan-17-one | HMDB | | 3b-Hydroxy-5a-androstane-17-one | HMDB | | 3b-Hydroxyandrostan-17-one | HMDB | | 3b-Hydroxyetioallocholan-17-one | HMDB | | 3b-OH-5a-Androstane-17-one | HMDB | | 5a-Androstan-3b-ol-17-one | HMDB | | 5a-Androstane-3b-ol-17-one | HMDB | | D-Epiandrosterone | HMDB | | Epi-andosterone | HMDB | | Epi-androsterone | HMDB | | Iso-androsterone | HMDB | | Isoandrosterone | HMDB | | trans-Androsterone | HMDB | | (3Β,5α)-3-hydroxyandrostan-17-one | HMDB | | 3Β-androsterone | HMDB | | 3Β-hydroxy-17-oxo-5α-androstane | HMDB | | 3Β-hydroxy-5α-androstan-17-one | HMDB | | 3Β-hydroxy-5α-androstane-17-one | HMDB | | 3Β-hydroxyandrostan-17-one | HMDB | | 3Β-hydroxyetioallocholan-17-one | HMDB | | 3Β-OH-5α-androstane-17-one | HMDB | | 5Α-androstan-17-one-3β-ol | HMDB | | 5Α-androstan-3β-ol-17-one | HMDB | | 5Α-androstane-3β-ol-17-one | HMDB | | (3beta,5alpha)-3-Hydroxyandrostan-17-one | HMDB | | 3beta-Androsterone | HMDB | | 3beta-Hydroxy-17-oxo-5alpha-androstane | HMDB | | 3beta-Hydroxy-5alpha-androstan-17-one | HMDB | | 3beta-Hydroxy-5alpha-androstane-17-one | HMDB | | 3beta-Hydroxyandrostan-17-one | HMDB | | 3beta-Hydroxyetioallocholan-17-one | HMDB | | 3beta-OH-5alpha-Androstane-17-one | HMDB | | 5alpha-Androstan-17-one-3beta-ol | HMDB | | 5alpha-Androstan-3beta-ol-17-one | HMDB | | 5alpha-Androstane-3beta-ol-17-one | HMDB |
|
|---|
| Chemical Formula | C19H30O2 |
|---|
| Average Molecular Weight | 290.4403 |
|---|
| Monoisotopic Molecular Weight | 290.224580204 |
|---|
| IUPAC Name | (1S,2S,5S,10R,11S,15S)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-one |
|---|
| Traditional Name | (1S,2S,5S,10R,11S,15S)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-one |
|---|
| CAS Registry Number | 481-29-8 |
|---|
| SMILES | [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2C[C@@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12-16,20H,3-11H2,1-2H3/t12?,13-,14-,15-,16-,18-,19-/m0/s1 |
|---|
| InChI Key | QGXBDMJGAMFCBF-QRIARFFBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- 3-hydroxysteroid
- 3-beta-hydroxysteroid
- Oxosteroid
- 17-oxosteroid
- Hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Endoplasmic reticulum
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 178 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.0202 mg/mL at 23 °C | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03ea-0390000000-17f2f9f14aa5e282a19a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0f7k-1139000000-2bff067a4137502d6ea7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-05fr-0190000000-b665b61b3fc9060b35b5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0a4i-4900000000-e43c3bdb0c139ec15f8b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-05mo-9300000000-c77bd97ae174005bf348 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-0090000000-fb385ad40b5d4ae1a6d9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dm-0290000000-6287ec557af150ef07fe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000t-3690000000-da6f3211b1e397dcf1ae | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-03f14a475acfab5558d6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-5e4902f7af7820145954 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06xx-2090000000-97cca29f0df7b3cc3714 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-afb176951034ef62419f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052e-0930000000-1ed776267953f7acda8a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-2900000000-1ba60805778f2942bfd7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-c8c452fd99c6bb4f3631 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-c8c452fd99c6bb4f3631 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0090000000-00c0e7de4e528b98df5c | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|