| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:29:31 UTC |
|---|
| Update Date | 2020-05-11 20:36:53 UTC |
|---|
| BMDB ID | BMDB0000372 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 16-Oxoestrone |
|---|
| Description | 16-Oxoestrone belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. Based on a literature review a significant number of articles have been published on 16-Oxoestrone. |
|---|
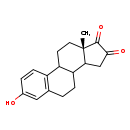
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 16-Ketoestrone | HMDB | | 3-Hydroxy-1,3,5(10)-estratriene-16,17-dione | HMDB |
|
|---|
| Chemical Formula | C18H20O3 |
|---|
| Average Molecular Weight | 284.3496 |
|---|
| Monoisotopic Molecular Weight | 284.141244506 |
|---|
| IUPAC Name | (15S)-5-hydroxy-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2(7),3,5-triene-13,14-dione |
|---|
| Traditional Name | (15S)-5-hydroxy-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2(7),3,5-triene-13,14-dione |
|---|
| CAS Registry Number | 1228-73-5 |
|---|
| SMILES | C[C@]12CCC3C(CCC4=C3C=CC(O)=C4)C1CC(=O)C2=O |
|---|
| InChI Identifier | InChI=1S/C18H20O3/c1-18-7-6-13-12-5-3-11(19)8-10(12)2-4-14(13)15(18)9-16(20)17(18)21/h3,5,8,13-15,19H,2,4,6-7,9H2,1H3/t13?,14?,15?,18-/m0/s1 |
|---|
| InChI Key | ANPHVANSJXDRTP-VLVBFLKRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-hydroxysteroid
- Hydroxysteroid
- 16-oxosteroid
- 17-oxosteroid
- Oxosteroid
- Phenanthrene
- Tetralin
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Ketone
- Cyclic ketone
- Carbonyl group
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 231 - 235 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ab9-0970000000-c33450029c2a510fb1dd | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0032-1095000000-28ce684570c9033f6ecb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-6d759bc421714d0d2920 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-0390000000-d8c66380533ac80c418f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gdi-7980000000-4a05c980006c31f9644c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-71415abfa8ce5bdff3d3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0090000000-66dbf6c2ee8a27f150f2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gdi-1090000000-dd632ccd4b6912edb4b7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-78a425e25a58a7330ede | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0090000000-ea975f2e73cc3dfd69ff | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01sl-0390000000-45c573c062efce8ba045 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-5bc7bcd7c5f268094120 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004r-0590000000-13c3647f4ba51b6998ee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-1790000000-7983b1aed115bbdf442d | View in MoNA |
|---|
|
|---|