| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:37:59 UTC |
|---|
| Update Date | 2020-04-22 15:05:45 UTC |
|---|
| BMDB ID | BMDB0000879 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Tetrahydrodeoxycorticosterone |
|---|
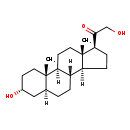
| Description | Tetrahydrodeoxycorticosterone, also known as 5alpha-thdoc or 5α-thdoc, belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. Based on a literature review a significant number of articles have been published on Tetrahydrodeoxycorticosterone. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5alpha-THDOC | Kegg | | 3alpha,21-Dihydroxy-5alpha-pregnan-20-one | Kegg | | 5a-THDOC | Generator | | 5Α-thdoc | Generator | | 3a,21-Dihydroxy-5a-pregnan-20-one | Generator | | 3Α,21-dihydroxy-5α-pregnan-20-one | Generator | | 3alpha,21-Dihydroxy-5beta-pregnan-20-one | HMDB | | 5-alpha-THDOC | HMDB | | Allotetrahydrodeoxycorticosterone | HMDB | | Deoxycorticosterone-21-aminopropane | HMDB | | Tetrahydro-11-deoxycorticosterone | HMDB | | 3 alpha,21-Dihydroxy-5 beta-pregnan-20-one | HMDB | | 3 alpha,5 beta-Tetrahydrodeoxycorticosterone | HMDB | | 3 beta,5 alpha-Tetrahydrodeoxycorticosterone | HMDB | | 3,21-Dihydroxypregnan-20-one | HMDB | | 5alpha-Pregnan-3alpha,21-diol-20-one | HMDB | | 5alpha-Pregnane-3alpha,21-diol-20-one | HMDB | | THDOC | HMDB | | Pregnane-3,21-diol-20-one | HMDB | | Tetrahydrodeoxycorticosterone, (3alpha,5alpha)-isomer | HMDB | | Tetrahydrodeoxycorticosterone, (3beta,5alpha)-isomer | HMDB | | Tetrahydrodesoxycorticosterone | HMDB |
|
|---|
| Chemical Formula | C21H34O3 |
|---|
| Average Molecular Weight | 334.4929 |
|---|
| Monoisotopic Molecular Weight | 334.250794954 |
|---|
| IUPAC Name | 2-hydroxy-1-[(1S,2S,5R,7S,10R,11S,14S,15S)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]ethan-1-one |
|---|
| Traditional Name | tetrahydrodeoxycorticosterone |
|---|
| CAS Registry Number | 567-03-3 |
|---|
| SMILES | [H][C@@]12CC[C@H](C(=O)CO)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C[C@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H34O3/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19(24)12-22)21(16,2)10-8-17(15)20/h13-18,22-23H,3-12H2,1-2H3/t13-,14+,15-,16-,17-,18+,20-,21-/m0/s1 |
|---|
| InChI Key | CYKYBWRSLLXBOW-GDYGHMJCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Hydroxysteroids |
|---|
| Direct Parent | 21-hydroxysteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 21-hydroxysteroid
- Pregnane-skeleton
- 20-oxosteroid
- 3-hydroxysteroid
- 3-alpha-hydroxysteroid
- Oxosteroid
- Alpha-hydroxy ketone
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organooxygen compound
- Primary alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05r0-0196000000-64a1fd698ec4c3241ddd | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-03di-1110900000-7b3fa2dd630d949e0a05 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0039000000-fbe3bc4f80d70022eede | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014s-0196000000-a18bb17686131ac405ed | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-1191000000-3c4df4d65182d58267d4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-cfaa8c0de99a027f7eb7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kur-2049000000-5582f42ba1bd76b52de1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-4092000000-3511eadb036a8910b388 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-3b9bbceb7e7c6cb37f9a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f89-0019000000-f0001403bdba14e2bf6c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f89-0089000000-91f07645e31574724cf2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0019000000-c5d82d2f86c8ce78130a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014s-0955000000-8d4884a7a7fed4ace8d8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052b-4930000000-4187c9aebfbeff89d19e | View in MoNA |
|---|
|
|---|