| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:42:03 UTC |
|---|
| Update Date | 2020-05-19 22:00:58 UTC |
|---|
| BMDB ID | BMDB0001176 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Cytidine monophosphate N-acetylneuraminic acid |
|---|
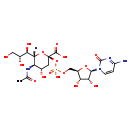
| Description | Cytidine monophosphate N-acetylneuraminic acid, also known as CMP acetylneuraminic acid or CMP-nana, belongs to the class of organic compounds known as pyrimidine nucleotide sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group. Cytidine monophosphate N-acetylneuraminic acid is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). Cytidine monophosphate N-acetylneuraminic acid exists in all living organisms, ranging from bacteria to humans. In cattle, cytidine monophosphate N-acetylneuraminic acid is involved in the metabolic pathway called the amino sugar metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| CMP Acetylneuraminic acid | ChEBI | | CMP-beta-Neu5ac | ChEBI | | CMP-N-Acetylneuraminate | ChEBI | | CMP-Nana | ChEBI | | CMP-Neu5ac | ChEBI | | CMP-NeuNAc | ChEBI | | CMP-Sialic acid | ChEBI | | Cytidine monophosphate N-acetylneuraminic acid | ChEBI | | CYTIDINE-5'-monophosphATE-5-N-acetylneuraminIC ACID | ChEBI | | Cytidine-5'-monophospho-N-acetylneuraminic acid | ChEBI | | Cytidine-5'-monophosphono-N-acetylneuraminic acid | ChEBI | | CMP-N-Acetyl-beta-neuraminate | Kegg | | CMP Acetylneuraminate | Generator | | CMP-b-Neu5ac | Generator | | CMP-Β-neu5ac | Generator | | CMP-N-Acetylneuraminic acid | Generator | | CMP-Sialate | Generator | | Cytidine monophosphate N-acetylneuraminate | Generator | | Cytidine monophosphoric acid N-acetylneuraminic acid | Generator | | CYTIDINE-5'-monophosphate-5-N-acetylneuraminate | Generator | | CYTIDINE-5'-monophosphoric acid-5-N-acetylneuraminic acid | Generator | | Cytidine-5'-monophospho-N-acetylneuraminate | Generator | | Cytidine-5'-monophosphono-N-acetylneuraminate | Generator | | CMP-N-Acetyl-b-neuraminate | Generator | | CMP-N-Acetyl-b-neuraminic acid | Generator | | CMP-N-Acetyl-beta-neuraminic acid | Generator | | CMP-N-Acetyl-β-neuraminate | Generator | | CMP-N-Acetyl-β-neuraminic acid | Generator | | Cytidine 5'-monophosphate-N-acetylneuraminate | Generator | | Cytidine 5'-monophosphoric acid-N-acetylneuraminic acid | Generator | | CMP-N-Acylneuraminate | HMDB | | Cytidine 5'-monophosphate N-acetylneuraminic acid | HMDB | | Acetylneuraminic acid, CMP | HMDB | | Acid, CMP acetylneuraminic | HMDB | | Acid, CMP-sialic | HMDB | | CMP Sialic acid | HMDB | | Cytidine monophosphate N acetylneuraminic acid | HMDB | | CMP-NeuAc | HMDB | | Cytidine 5'-monophosphic acid-N-acetylneuraminic acid | HMDB | | Cytidine 5'-monophospho-N-acetylneuraminate | HMDB | | Cytidine 5'-monophospho-N-acetylneuraminic acid | HMDB | | Cytidine 5’-monophosphate-N-acetylneuraminate | HMDB | | Cytidine 5’-monophosphate-N-acetylneuraminic acid | HMDB | | Cytidine 5’-monophosphic acid-N-acetylneuraminic acid | HMDB | | Cytidine 5’-monophospho-N-acetylneuraminate | HMDB | | Cytidine 5’-monophospho-N-acetylneuraminic acid | HMDB | | Cytidine monophosphic acid N-acetylneuraminic acid | HMDB | | Cytidine 5'-monophosphate-N-acetylneuraminic acid | HMDB |
|
|---|
| Chemical Formula | C20H31N4O16P |
|---|
| Average Molecular Weight | 614.4511 |
|---|
| Monoisotopic Molecular Weight | 614.147267476 |
|---|
| IUPAC Name | (2R,4S,5R,6R)-2-[({[(2R,3S,4R,5R)-5-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]-5-acetamido-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid |
|---|
| Traditional Name | cmp-sialic acid |
|---|
| CAS Registry Number | 22-12-8 |
|---|
| SMILES | [H][C@]1(O[C@](C[C@H](O)[C@H]1NC(C)=O)(OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=CC(N)=NC1=O)C(O)=O)[C@H](O)[C@H](O)CO |
|---|
| InChI Identifier | InChI=1S/C20H31N4O16P/c1-7(26)22-12-8(27)4-20(18(32)33,39-16(12)13(29)9(28)5-25)40-41(35,36)37-6-10-14(30)15(31)17(38-10)24-3-2-11(21)23-19(24)34/h2-3,8-10,12-17,25,27-31H,4-6H2,1H3,(H,22,26)(H,32,33)(H,35,36)(H2,21,23,34)/t8-,9+,10+,12+,13+,14+,15+,16+,17+,20+/m0/s1 |
|---|
| InChI Key | TXCIAUNLDRJGJZ-BILDWYJOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrimidine nucleotide sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Pyrimidine nucleotides |
|---|
| Sub Class | Pyrimidine nucleotide sugars |
|---|
| Direct Parent | Pyrimidine nucleotide sugars |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidine nucleotide sugar
- Pyrimidine ribonucleoside monophosphate
- N-acylneuraminic acid
- N-acylneuraminic acid or derivatives
- Neuraminic acid
- Pentose phosphate
- Pentose-5-phosphate
- C-glucuronide
- C-glycosyl compound
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Aminopyrimidine
- Pyrimidone
- Dialkyl phosphate
- Hydropyrimidine
- Monosaccharide
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Pyran
- Pyrimidine
- Imidolactam
- Alkyl phosphate
- Tetrahydrofuran
- Acetamide
- Heteroaromatic compound
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Amino acid
- Amino acid or derivatives
- Polyol
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Monocarboxylic acid or derivatives
- Organopnictogen compound
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Primary amine
- Alcohol
- Primary alcohol
- Hydrocarbon derivative
- Amine
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ot-9512160000-178f009287639a11dbfd | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-044l-9624017000-8c596d9b4644a97fbb65 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0912020000-9d330aadc1882c503212 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-3902000000-b2c85490ddb7f53db6f4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-6910000000-a8d1f94c4661e1dd3097 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-4813091000-d68fb03c5bbe9978f2e5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-4912010000-168821fb3a4dc5a309b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08fr-9310000000-5e2455827ac9cc8b0a87 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-2004169000-eac426191a551fa0ea5f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0bvs-9003180000-afd899893a04da29e2e4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-9001310000-a0291ac7ad84d4ba3ecb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0240369000-cae65d161602c1ecc345 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-1864591000-a0ca8cc5e78443e5226c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03k9-3943120000-4ac27539b86275883867 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|