| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:48:47 UTC |
|---|
| Update Date | 2020-04-22 15:09:03 UTC |
|---|
| BMDB ID | BMDB0001898 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Mesobilirubinogen |
|---|
| Description | Mesobilirubinogen, also known as i-urobilinogen or urobilinogen ixalpha, belongs to the class of organic compounds known as bilirubins. These are organic compounds containing a dicarboxylic acyclic tetrapyrrole derivative. Based on a literature review a significant number of articles have been published on Mesobilirubinogen. |
|---|
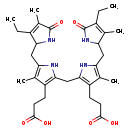
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,17-Diethyl-1,4,5,10,15,16,19,22,23,24-decahydro-3,7,13,18-tetramethyl-1,19-dioxo-21H-biline-8,12-dipropanoic acid | ChEBI | | 8,12-Bis(2-carboxyethyl)-3,18-diethyl-2,7,13,17-tetramethylbilane-1,19(4H,16H)-dione | ChEBI | | I-urobilinogen | ChEBI | | Mesobilirubinogen ixalpha | ChEBI | | Urobilinogen ixalpha | ChEBI | | Urobilinogen | Kegg | | 2,17-Diethyl-1,4,5,10,15,16,19,22,23,24-decahydro-3,7,13,18-tetramethyl-1,19-dioxo-21H-biline-8,12-dipropanoate | Generator | | 2,17-Diethyl-1,4,5,10,15,16,19,22,23,24-decahydro-3,7,13,18-tetramethyl-1,19-dioxo-biline-8,12-dipropionic acid | HMDB | | Mesobilirubinogen | ChEBI | | Mesobilirubinogen IXα | HMDB | | Urobilinogen IXα | HMDB |
|
|---|
| Chemical Formula | C33H44N4O6 |
|---|
| Average Molecular Weight | 592.7257 |
|---|
| Monoisotopic Molecular Weight | 592.32608516 |
|---|
| IUPAC Name | 3-(2-{[3-(2-carboxyethyl)-5-[(4-ethyl-3-methyl-5-oxo-2,5-dihydro-1H-pyrrol-2-yl)methyl]-4-methyl-1H-pyrrol-2-yl]methyl}-5-[(3-ethyl-4-methyl-5-oxo-2,5-dihydro-1H-pyrrol-2-yl)methyl]-4-methyl-1H-pyrrol-3-yl)propanoic acid |
|---|
| Traditional Name | urobilinogen |
|---|
| CAS Registry Number | 14684-37-8 |
|---|
| SMILES | CCC1=C(C)C(=O)NC1CC1=C(C)C(CCC(O)=O)=C(CC2=C(CCC(O)=O)C(C)=C(CC3NC(=O)C(CC)=C3C)N2)N1 |
|---|
| InChI Identifier | InChI=1S/C33H44N4O6/c1-7-20-19(6)32(42)37-27(20)14-25-18(5)23(10-12-31(40)41)29(35-25)15-28-22(9-11-30(38)39)17(4)24(34-28)13-26-16(3)21(8-2)33(43)36-26/h26-27,34-35H,7-15H2,1-6H3,(H,36,43)(H,37,42)(H,38,39)(H,40,41) |
|---|
| InChI Key | OBHRVMZSZIDDEK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bilirubins. These are organic compounds containing a dicarboxylic acyclic tetrapyrrole derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Bilirubins |
|---|
| Direct Parent | Bilirubins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bilirubin skeleton
- Dicarboxylic acid or derivatives
- Substituted pyrrole
- Pyrrole
- Pyrroline
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Lactam
- Carboxamide group
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Organopnictogen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 1.969 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-6500190000-673e8c4460e3cdbdd8e2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dj-7500019000-dc792020abb23fbb3064 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Mesobilirubinogen,1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_15) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT , negative | splash10-014i-0060900000-67682461aceaa81b9cc2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-006x-0000090000-a65bbba494a7e099f9c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dm-1100190000-7cf7854534fedc5bbfc1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9010240000-d75cd70ca41532190bfc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000290000-a18b99b2b4373146a032 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-2200890000-cf0c2e6ff644591038b0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000f-4970330000-d515de7fece55949f271 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000090000-181b914f66e7110dfe7d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00bj-0100290000-875b38ee4fed5fc0ecd8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05n0-1010940000-9f8c080267aa82fc0b96 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ufr-0011960000-50b1dbb2c2c4f704b5c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0w4i-0210940000-17857bfc32a5c7d5f66c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-008a-4642920000-dd8a11d07863f98ea6f2 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Watson, C. J.; Lightner, D. A.; Moscowitz, A.; Davis, E.; Petryka, Z. J.; Weimer, M. A natural crystalline urobilinogen composed of d- and l-components of differing molecular weight. Proceedings of the National Academy of Sciences of the United States of America (1968), 61(1), 223-8 |
|---|