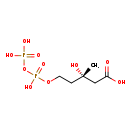| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:49:31 UTC |
|---|
| Update Date | 2020-05-11 20:19:52 UTC |
|---|
| BMDB ID | BMDB0001981 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | (R)-Mevalonic acid-5-pyrophosphate |
|---|
| Description | (R)-Mevalonic acid-5-pyrophosphate belongs to the class of organic compounds known as organic pyrophosphates. These are organic compounds containing the pyrophosphate oxoanion, with the structure OP([O-])(=O)OP(O)([O-])=O. Based on a literature review a significant number of articles have been published on (R)-Mevalonic acid-5-pyrophosphate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R)-Mevalonate-5-pyrophosphate | Generator | | (R)-Mevalonic acid-5-pyrophosphoric acid | Generator | | (R)-5-Diphosphomevalonic acid | HMDB | | 1,1,3,7-Tetrahydroxy-7-methyl-2,4-dioxa-1,3-diphosphanonan-9-Oate | HMDB | | 1,1,3,7-Tetrahydroxy-7-methyl-2,4-dioxa-1,3-diphosphanonan-9-Oic acid | HMDB | | 1,1,3,7-Tetrahydroxy-7-methyl-2,4-dioxa-1,3-diphosphanonan-9-Oic acid 1,3-dioxide | HMDB | | 5-Pyrophosphomevalonate | HMDB | | 5-Pyrophosphomevalonic acid | HMDB | | Mevalonate 5-diphosphate | HMDB | | Mevalonic 5-pyrophosphate | HMDB | | Mevalonic acid 5-diphosphate | HMDB | | Mevalonic acid 5-pyrophosphate | HMDB | | Mevalonic acid pyrophosphate | HMDB | | MVADP | HMDB | | Pyrophosphomevalonate | HMDB | | Pyrophosphomevalonic acid | HMDB | | R-Mevalonic acid-5-pyrophosphate | HMDB | | Mevalonate pyrophosphate | MeSH, HMDB | | 5-Diphosphomevalonic acid | MeSH, HMDB | | (3S)-3-Hydroxy-5-{[hydroxy(phosphonooxy)phosphoryl]oxy}-3-methylpentanoate | Generator, HMDB |
|
|---|
| Chemical Formula | C6H14O10P2 |
|---|
| Average Molecular Weight | 308.1169 |
|---|
| Monoisotopic Molecular Weight | 308.006219692 |
|---|
| IUPAC Name | (3S)-3-hydroxy-5-{[hydroxy(phosphonooxy)phosphoryl]oxy}-3-methylpentanoic acid |
|---|
| Traditional Name | pyrophosphomevalonate |
|---|
| CAS Registry Number | 1492-08-6 |
|---|
| SMILES | C[C@](O)(CCOP(O)(=O)OP(O)(O)=O)CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H14O10P2/c1-6(9,4-5(7)8)2-3-15-18(13,14)16-17(10,11)12/h9H,2-4H2,1H3,(H,7,8)(H,13,14)(H2,10,11,12)/t6-/m0/s1 |
|---|
| InChI Key | SIGQQUBJQXSAMW-LURJTMIESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as organic pyrophosphates. These are organic compounds containing the pyrophosphate oxoanion, with the structure OP([O-])(=O)OP(O)([O-])=O. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organic oxoanionic compounds |
|---|
| Sub Class | Organic pyrophosphates |
|---|
| Direct Parent | Organic pyrophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic pyrophosphate
- Monoalkyl phosphate
- Short-chain hydroxy acid
- Organic phosphoric acid derivative
- Fatty acid
- Alkyl phosphate
- Phosphoric acid ester
- Tertiary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Carbonyl group
- Alcohol
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004m-9820000000-20a5a060549dcb7bd8d3 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00dv-9243200000-6d8177d69adb810da37f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 5V, negative | splash10-000i-0090000000-27d85eaaa9c4e19f0fcf | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 6V, negative | splash10-000i-0090000000-8e9a7018cd2663189521 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 9V, negative | splash10-002r-3490000000-b2f4bcb965dfbe91ce8f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 11V, negative | splash10-004r-7690000000-533cfa9b6449fe59e31d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 12V, negative | splash10-004i-9540000000-ba3a429bcc345a49d5ff | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 15V, negative | splash10-004i-9310000000-b552a514df86bf203483 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 18V, negative | splash10-004i-9200000000-73f02610e11b09ea0ae0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 20V, negative | splash10-004i-9100000000-e99ccf8685bbb8c58b7f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 25V, negative | splash10-004i-9000000000-df1600382f0903920e95 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 21V, negative | splash10-004i-1900000000-0d135499da95ac5499d5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 21V, negative | splash10-0a4i-0900000000-b559d4679fc0eaa79be7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 21V, negative | splash10-004i-9000000000-46d4cee1b5ac630ba9b8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 21V, negative | splash10-004i-1590000000-bd14b17224627a5d2418 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 21V, negative | splash10-0a4i-0900000000-dc954ce23ecf1a263189 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 21V, negative | splash10-0kdi-0490000000-356afce14b98128806e5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 5V, negative | splash10-0a4i-0009000000-ece59023c1177bedb2f3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 7V, negative | splash10-0a4i-0009000000-cbc9841391b1e5bea83c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 9V, negative | splash10-0a4i-1109000000-71626d1a601d118a3d29 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 11V, negative | splash10-0a6r-9516000000-b1e714407672ea74b65f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 14V, negative | splash10-004i-9300000000-79a6a45a050a4557acba | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 17V, negative | splash10-004i-9100000000-53b8fc8a95948a4c9764 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 20V, negative | splash10-004i-9100000000-0afb1187a03463994c7a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 24V, negative | splash10-004i-9000000000-92672b421a976d0722e3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 28V, negative | splash10-004i-9000000000-ad774759f01ecd022463 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 33V, negative | splash10-004i-9000000000-028cfb3af846dc9c0ee5 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|