| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:52:13 UTC |
|---|
| Update Date | 2020-04-22 15:10:03 UTC |
|---|
| BMDB ID | BMDB0002181 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
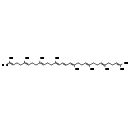
| Common Name | Phytoene |
|---|
| Description | Phytoene, also known as (all-e)-phytoene or trans-phytoene, belongs to the class of organic compounds known as carotenes. These are a type of unsaturated hydrocarbons containing eight consecutive isoprene units. They are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Thus, phytoene is considered to be an isoprenoid. Based on a literature review a significant number of articles have been published on Phytoene. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (6E,10E,14E,16E,18E,22E,26E)-2,6,10,14,19,23,27,31-Octamethyldotriaconta-2,6,10,14,16,18,22,26,30-nonaene | ChEBI | | 7,7',8,8',11,11',12,12'-Octahydro-psi,psi-carotene | ChEBI | | (all-e)-Phytoene | HMDB | | all-trans-7,7',8,8',11,11',12,12'-Octahydro-lycopene | HMDB | | all-trans-Phytoene | HMDB | | trans-Phytoene | HMDB | | (all-e) Phytoene | HMDB | | 7,7',8,8',11,11',12,12'-Octahydro-ψ,ψ-carotene | HMDB | | 7,7’,8,8’,11,11’,12,12’-octahydro-ψ,ψ-carotene | HMDB |
|
|---|
| Chemical Formula | C40H64 |
|---|
| Average Molecular Weight | 544.9362 |
|---|
| Monoisotopic Molecular Weight | 544.500802048 |
|---|
| IUPAC Name | (6E,10E,14E,16E,18E,22E,26E)-2,6,10,14,19,23,27,31-octamethyldotriaconta-2,6,10,14,16,18,22,26,30-nonaene |
|---|
| Traditional Name | phytoene |
|---|
| CAS Registry Number | 540-04-5 |
|---|
| SMILES | CC(C)=CCC\C(C)=C\CC\C(C)=C\CC\C(C)=C\C=C\C=C(/C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C40H64/c1-33(2)19-13-23-37(7)27-17-31-39(9)29-15-25-35(5)21-11-12-22-36(6)26-16-30-40(10)32-18-28-38(8)24-14-20-34(3)4/h11-12,19-22,27-30H,13-18,23-26,31-32H2,1-10H3/b12-11+,35-21+,36-22+,37-27+,38-28+,39-29+,40-30+ |
|---|
| InChI Key | YVLPJIGOMTXXLP-KEKOKYSKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as carotenes. These are a type of unsaturated hydrocarbons containing eight consecutive isoprene units. They are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Carotenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Carotene
- Branched unsaturated hydrocarbon
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Acyclic olefin
- Hydrocarbon
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0570-6737950000-7711f2e47eaad6db7dec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0221190000-3244d5283223f5646dea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a73-2984620000-0979dace9bacc0f9a33c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4r-3777900000-f7a566e5a1a7d0e85965 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000090000-5470ec221f6475188119 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0000090000-0fab68faee101ed9388b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-1964680000-912b2d47f235939dc5c7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000090000-a09e228124f7e70550ec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0130190000-51d2c39c749d50842ec5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-1301910000-b874116ff6c9bd752bf0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-2450690000-e405778b880bd192827d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fsr-2100920000-e081fc96fa43a10f0504 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01zi-1423910000-2025343822b18280fa44 | View in MoNA |
|---|
|
|---|