| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:55:24 UTC |
|---|
| Update Date | 2020-04-22 15:11:01 UTC |
|---|
| BMDB ID | BMDB0002451 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 7b,12a-Dihydroxycholanoic acid |
|---|
| Description | 7b,12a-Dihydroxycholanoic acid belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. Based on a literature review a significant number of articles have been published on 7b,12a-Dihydroxycholanoic acid. |
|---|
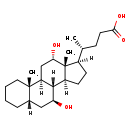
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7b,12a-Dihydroxycholanoate | Generator | | (5b,7b,12a)-7,12-Dihydroxy-cholan-24-Oate | HMDB | | (5b,7b,12a)-7,12-Dihydroxy-cholan-24-Oic acid | HMDB | | 7b,12a-Dihydroxy-5b-cholan-24-Oate | HMDB | | 7b,12a-Dihydroxy-5b-cholan-24-Oic acid | HMDB | | 7b,12a-Dihydroxy-5b-cholanoate | HMDB | | 7b,12a-Dihydroxy-5b-cholanoic acid | HMDB | | 7beta,12alpha-Dihydroxycholanoate | HMDB | | 7beta,12alpha-Dihydroxycholanoic acid | HMDB | | (4R)-4-[(1S,2S,7S,9S,10R,11S,14R,15R,16S)-9,16-Dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoate | Generator, HMDB |
|
|---|
| Chemical Formula | C24H40O4 |
|---|
| Average Molecular Weight | 392.572 |
|---|
| Monoisotopic Molecular Weight | 392.292659768 |
|---|
| IUPAC Name | (4R)-4-[(1S,2S,7S,9S,10R,11S,14R,15R,16S)-9,16-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoic acid |
|---|
| Traditional Name | 7β,12α-dihydroxycholanoic acid |
|---|
| CAS Registry Number | 84413-81-0 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])CCCC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C24H40O4/c1-14(7-10-21(27)28)16-8-9-17-22-18(13-20(26)24(16,17)3)23(2)11-5-4-6-15(23)12-19(22)25/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17+,18+,19+,20+,22+,23+,24-/m1/s1 |
|---|
| InChI Key | ZHCAAZIHTDCFJX-BJMHMCHXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydroxy bile acids, alcohols and derivatives. Dihydroxy bile acids, alcohols and derivatives are compounds containing or derived from a bile acid or alcohol, and which bears exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Dihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydroxy bile acid, alcohol, or derivatives
- 12-hydroxysteroid
- Hydroxysteroid
- 7-alpha-hydroxysteroid
- 7-hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Alcohol
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01r2-1349000000-c20944bdb515b39b1d8b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0006-2110290000-a65713723f244ae437ac | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0009000000-581fe3701cfd4409cf1c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-1009000000-5e9bdccb7f6cc0d63c63 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02bb-6009000000-2e184bcce3eab0a12b14 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-006x-0009000000-f969293bf286459bff3f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dl-0009000000-6d3e401b3a1ad3688eb2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9007000000-35f153d5acdaba8f3b23 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-e572c29938091d5ba610 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05bf-5297000000-e9bd316f7bfb3538706a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052b-9530000000-343097ad815cfbd487e1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-7eba834115ee2c679208 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-0009000000-03834bc03b5a1c28e5f1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-1039000000-b5eba12dd80348c13a4d | View in MoNA |
|---|
|
|---|