| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:01:28 UTC |
|---|
| Update Date | 2020-05-11 20:23:23 UTC |
|---|
| BMDB ID | BMDB0002728 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Thyroxine sulfate |
|---|
| Description | Thyroxine sulfate, also known as T4S or T4 sulfate, belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Thyroxine sulfate is a very strong basic compound (based on its pKa). |
|---|
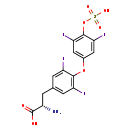
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Thyroxine sulfuric acid | Generator | | Thyroxine sulphate | Generator | | Thyroxine sulphuric acid | Generator | | 3,5,3',5'-Tetraiodo-L-thyronine 4'-O-sulfate | HMDB | | 3,5,3',5'-Tetraiodo-L-thyronine 4'-O-sulphate | HMDB | | 3,5,3',5'-Tetraiodo-L-thyronine 4-O-sulfate | HMDB | | 3,5,3',5'-Tetraiodo-L-thyronine 4-O-sulphate | HMDB | | 3-[4-(4-Hydroxy-3,5-diiodophenoxy-4-O-sulfate)-3,5-diiodophenyl]-L-alanine | HMDB | | 3-[4-(4-Hydroxy-3,5-diiodophenoxy-4-O-sulphate)-3,5-diiodophenyl]-L-alanine | HMDB | | L-Thyroxine 4'-O-sulfate | HMDB | | L-Thyroxine 4'-O-sulphate | HMDB | | O-(4-Hydroxy-3,5-diiodophenyl-4-O-sulfate)-3,5-diiodo-L-tyrosine | HMDB | | O-(4-Hydroxy-3,5-diiodophenyl-4-O-sulphate)-3,5-diiodo-L-tyrosine | HMDB | | T4S | HMDB | | Thyroxine-4-sulfate | HMDB | | T4 Sulfate | HMDB | | (2S)-2-Amino-3-{4-[3,5-diiodo-4-(sulfooxy)phenoxy]-3,5-diiodophenyl}propanoate | HMDB | | (2S)-2-Amino-3-{4-[3,5-diiodo-4-(sulphooxy)phenoxy]-3,5-diiodophenyl}propanoate | HMDB | | (2S)-2-Amino-3-{4-[3,5-diiodo-4-(sulphooxy)phenoxy]-3,5-diiodophenyl}propanoic acid | HMDB | | Thyroxine sulfate | MeSH |
|
|---|
| Chemical Formula | C15H11I4NO7S |
|---|
| Average Molecular Weight | 856.933 |
|---|
| Monoisotopic Molecular Weight | 856.643496081 |
|---|
| IUPAC Name | (2S)-2-amino-3-{4-[3,5-diiodo-4-(sulfooxy)phenoxy]-3,5-diiodophenyl}propanoic acid |
|---|
| Traditional Name | thyroxine sulfic acid |
|---|
| CAS Registry Number | 77074-49-8 |
|---|
| SMILES | N[C@@H](CC1=CC(I)=C(OC2=CC(I)=C(OS(O)(=O)=O)C(I)=C2)C(I)=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C15H11I4NO7S/c16-8-1-6(3-12(20)15(21)22)2-9(17)13(8)26-7-4-10(18)14(11(19)5-7)27-28(23,24)25/h1-2,4-5,12H,3,20H2,(H,21,22)(H,23,24,25)/t12-/m0/s1 |
|---|
| InChI Key | QYXIJUZWSSQICT-LBPRGKRZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Phenylalanine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylalanine or derivatives
- Diphenylether
- 3-phenylpropanoic-acid
- Phenylsulfate
- Diaryl ether
- Alpha-amino acid
- Amphetamine or derivatives
- L-alpha-amino acid
- Arylsulfate
- Phenoxy compound
- Phenol ether
- Halobenzene
- Aralkylamine
- Iodobenzene
- Monocyclic benzene moiety
- Benzenoid
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- Aryl halide
- Aryl iodide
- Organic sulfuric acid or derivatives
- Amino acid
- Carboxylic acid
- Ether
- Monocarboxylic acid or derivatives
- Primary aliphatic amine
- Organonitrogen compound
- Organooxygen compound
- Amine
- Primary amine
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organohalogen compound
- Organoiodide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-08fu-0000000190-60a2d3c20ec6cb9d1c69 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ec-0000000960-1acc6f8ba4fa640eea14 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-016r-0009201200-f3a8fb5f5d259b4a08de | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000000190-dcd5150fd64eb9666b36 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-1001200930-81382d7ac99b1b206504 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00am-9004204800-333dbdf28ab736330d4a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000000090-d55170c46c67ae342e9e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-2800000190-20c0887d6c545eb80a28 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0900000000-0dba40d354fa6a5575e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000000090-0f9cdad016a751aa1ba6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000000290-c5f42e3c3ab1663956b8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fai-0000000900-4ef5f51f90403aad561a | View in MoNA |
|---|
|
|---|