| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:01:50 UTC |
|---|
| Update Date | 2020-04-22 15:11:27 UTC |
|---|
| BMDB ID | BMDB0002835 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Danazol |
|---|
| Description | Danazol, also known as danocrine or cyclomen, belongs to the class of organic compounds known as estrane steroids. These are steroids with a structure based on the estrane skeleton. Danazol is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
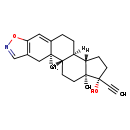
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cyclomen | ChEBI | | Danazolum | ChEBI | | Danocrine | ChEBI | | (17a)-Pregna-2,4-dien-20-yno[2,3-D]isoxazol-17-ol | HMDB | | 1-Ethynyl-2,3,3a,3b,4,5,10,10a,10b,11,12,12a-dodecahydro-10a,12a-dimethyl-1H-cyclopenta[7,8]phenanthro[3,2-D]isoxazol-1-ol | HMDB | | 17 alpha-Pregna-2,4-dien-20-yno[2,3-D] isoxazol-17 beta-ol | HMDB | | 17a-Pregna-2,4-dien-20-yne-[2,3-D]isoxazole-17b-ol | HMDB | | 17a-Pregna-2,4-dien-20-yno[2,3-D]isoxazol-17-ol | HMDB | | 1H-Cyclopenta[7,8]phenanthro[3,2-D]isoxazole- pregna-2,4-dien-20-yno[2,3-D]isoxazol-17-ol deriv. | HMDB | | Bonzol | HMDB | | Chronogyn | HMDB | | Danol | HMDB | | Danovaol | HMDB | | Danzol | HMDB | | Ladogal | HMDB | | Winobanin | HMDB | | Alphapharm brand OF danazol | HMDB | | Kendrick brand OF danazol | HMDB | | Sanofi brand OF danazol | HMDB | | Danatrol | HMDB | | Danazant | HMDB | | Danazol-ratiopharm | HMDB | | Danoval | HMDB | | Ratiopharm brand OF danazol | HMDB | | Antigen brand OF danazol | HMDB | | Azol | HMDB | | Norciden | HMDB | | Panacrine | HMDB | | Sanofi synthelabo brand OF danazol | HMDB | | Sanofi winthrop brand OF danazol | HMDB | | Danazol ratiopharm | HMDB |
|
|---|
| Chemical Formula | C22H27NO2 |
|---|
| Average Molecular Weight | 337.4553 |
|---|
| Monoisotopic Molecular Weight | 337.204179113 |
|---|
| IUPAC Name | (1S,2R,13R,14S,17R,18S)-17-ethynyl-2,18-dimethyl-7-oxa-6-azapentacyclo[11.7.0.0^{2,10}.0^{4,8}.0^{14,18}]icosa-4(8),5,9-trien-17-ol |
|---|
| Traditional Name | (1S,2R,13R,14S,17R,18S)-17-ethynyl-2,18-dimethyl-7-oxa-6-azapentacyclo[11.7.0.0^{2,10}.0^{4,8}.0^{14,18}]icosa-4(8),5,9-trien-17-ol |
|---|
| CAS Registry Number | 17230-88-5 |
|---|
| SMILES | [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC3=C(C[C@]12C)C=NO3 |
|---|
| InChI Identifier | InChI=1S/C22H27NO2/c1-4-22(24)10-8-18-16-6-5-15-11-19-14(13-23-25-19)12-20(15,2)17(16)7-9-21(18,22)3/h1,11,13,16-18,24H,5-10,12H2,2-3H3/t16-,17+,18+,20+,21+,22+/m1/s1 |
|---|
| InChI Key | POZRVZJJTULAOH-LHZXLZLDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrane steroids. These are steroids with a structure based on the estrane skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrane steroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrane-skeleton
- Hydroxysteroid
- 17-hydroxysteroid
- Ynone
- Heteroaromatic compound
- Azole
- Cyclic alcohol
- Tertiary alcohol
- Isoxazole
- Acetylide
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 225.6 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 4.081 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ab9-0669000000-7afc135d5b1e92134191 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-000x-2479000000-d5f94bb7e7215a30f820 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-0009000000-9e745b8feecd354f6618 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-01b9-6902000000-854476472755ef5419e5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0006-9100000000-0d62c0c1b53c1cea5a25 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-000i-0329000000-56adc37fd83dfa3c686f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-05ia-3931000000-1490f6c4984a84016076 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-002f-6900000000-529d2d24864b26fdd611 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-002f-6900000000-01f21f6ea357e6649d15 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-000i-1309000000-271d4ce8113e1bf16016 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-00dj-2910000000-3af637f5ddbf8cfd6b84 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-00dj-2920000000-ba6bb4b948be2171d864 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-05tg-3900000000-29df3c1f08b04956955d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-000i-0009000000-5fc69729a45a40e94073 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-002f-4900000000-7ba349192301fd4e5013 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-05tg-3900000000-a692616aee182eeb1270 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0119000000-d7acb78e38c02e285d4f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01wr-0289000000-2db311b410937e72f25a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000j-3690000000-5eb98df42b0016f9967a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-d1a89ea3e6c72ff1ab88 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-71fbd4000b06296a8386 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00e9-2089000000-24b6d7f3ec074a6c2b59 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0019000000-2626c30d979a970252f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-007y-0982000000-4cf29ec44560862d68c7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-1001-1890000000-24565065bfdf6e8edf39 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-8413c28ca2b1374741c7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-3424fea43411af836104 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Raczkowska, Sabina; Lypacewicz, Maria K.; Chojecka-Koryn, Ewa; Jaworska, Romana; Wasiak, Teresa; Mozolowski, Felicjan; Wajcht, Jozef. Method of obtaining highly pure danazol. Pol. (1991), 3 pp. |
|---|