| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:02:38 UTC |
|---|
| Update Date | 2020-05-11 20:56:05 UTC |
|---|
| BMDB ID | BMDB0003069 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 20a-Dihydroprogesterone |
|---|
| Description | 20a-Dihydroprogesterone, also known as 20b-hydroxy-4-pregnen-3-one or 20-alpha-progerol, belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. Thus, 20a-dihydroprogesterone is considered to be a steroid lipid molecule. 20a-Dihydroprogesterone is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. |
|---|
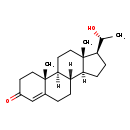
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 20beta-Dihydroprogesterone | ChEBI | | 20beta-Hydroxy-4-pregnen-3-one | ChEBI | | 20beta-Hydroxypregn-4-en-3-one | ChEBI | | 20b-Dihydroprogesterone | Generator | | 20Β-dihydroprogesterone | Generator | | 20b-Hydroxy-4-pregnen-3-one | Generator | | 20Β-hydroxy-4-pregnen-3-one | Generator | | 20b-Hydroxypregn-4-en-3-one | Generator | | 20Β-hydroxypregn-4-en-3-one | Generator | | Dihydrogesterone | HMDB | | Dihydroxyprogesterone | HMDB | | Dihydroprogesterone | HMDB | | Algestone | HMDB |
|
|---|
| Chemical Formula | C21H32O2 |
|---|
| Average Molecular Weight | 316.4776 |
|---|
| Monoisotopic Molecular Weight | 316.240230268 |
|---|
| IUPAC Name | (1S,2R,10S,11S,14S,15S)-14-[(1R)-1-hydroxyethyl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-5-one |
|---|
| Traditional Name | 20β-dihydroprogesterone |
|---|
| CAS Registry Number | 145-14-2 |
|---|
| SMILES | [H][C@@]12CC[C@H]([C@@H](C)O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H32O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h12-13,16-19,22H,4-11H2,1-3H3/t13-,16+,17-,18+,19+,20+,21-/m1/s1 |
|---|
| InChI Key | RWBRUCCWZPSBFC-SJOKZOANSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-hydroxysteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Cyclic ketone
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | - 20-hydroxypregn-4-en-3-one (CHEBI:36729 )
- C21 steroids (gluco/mineralocorticoids, progestogins) and derivatives (LMST02030153 )
|
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 126 - 131 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | |
|---|