| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:03:24 UTC |
|---|
| Update Date | 2020-04-22 15:11:55 UTC |
|---|
| BMDB ID | BMDB0003263 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Pelargonidin |
|---|
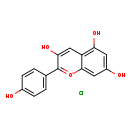
| Description | 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-1λ⁴-chromen-1-ylium, also known as 3,4',5,7-tetrahydroxyflavylium chloride or pelargonidol chloride, belongs to the class of organic compounds known as 7-hydroxyflavonoids. These are flavonoids that bear one hydroxyl group at the C-7 position of the flavonoid skeleton. 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-1λ⁴-chromen-1-ylium is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,4',5,7-Tetrahydroxyflavylium chloride | ChEBI | | 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-1-benzopyrylium chloride | ChEBI | | 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)benzopyrylium chloride | ChEBI | | Pelargonidin | ChEBI | | Pelargonidol chloride | ChEBI | | 3,4',5,7-Tetrahydroxy-flavylium | HMDB | | 3,4',5,7-Tetrahydroxyflavylium | HMDB | | 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-1-benzopyrylium | HMDB | | Pelargonidol | HMDB | | Pelargonidine | HMDB |
|
|---|
| Chemical Formula | C15H11ClO5 |
|---|
| Average Molecular Weight | 306.698 |
|---|
| Monoisotopic Molecular Weight | 306.029501169 |
|---|
| IUPAC Name | 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-1lambda4-chromen-1-ylium chloride |
|---|
| Traditional Name | 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-1lambda4-chromen-1-ylium chloride |
|---|
| CAS Registry Number | 134-04-3 |
|---|
| SMILES | [Cl-].OC1=CC=C(C=C1)C1=[O+]C2=CC(O)=CC(O)=C2C=C1O |
|---|
| InChI Identifier | InChI=1S/C15H10O5.ClH/c16-9-3-1-8(2-4-9)15-13(19)7-11-12(18)5-10(17)6-14(11)20-15;/h1-7H,(H3-,16,17,18,19);1H |
|---|
| InChI Key | YPVZJXMTXCOTJN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 7-hydroxyflavonoids. These are flavonoids that bear one hydroxyl group at the C-7 position of the flavonoid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Hydroxyflavonoids |
|---|
| Direct Parent | 7-hydroxyflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- 7-hydroxyflavonoid
- Anthocyanidin
- Benzopyran
- 1-benzopyran
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Heteroaromatic compound
- Organoheterocyclic compound
- Oxacycle
- Organooxygen compound
- Hydrochloride
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic zwitterion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | > 350 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | |
|---|