| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:05:24 UTC |
|---|
| Update Date | 2020-05-11 20:30:57 UTC |
|---|
| BMDB ID | BMDB0003573 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Scopolamine |
|---|
| Description | Scopolamine, also known as hyoscine or transderm-scop, belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. Based on a literature review a significant number of articles have been published on Scopolamine. |
|---|
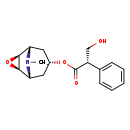
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Hyoscine | ChEBI | | (-)-Scopolamine | ChEBI | | (1S,3S,5R,6R,7S)-6,7-Epoxytropan-3-yl (2S)-3-hydroxy-2-phenylpropanoate | ChEBI | | 6,7-Epoxytropine tropate | ChEBI | | 6-beta,7-beta-Epoxy-3-alpha-tropanyl S-(-)-tropate | ChEBI | | alpha-(Hydroxymethyl)benzeneacetic acid 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl ester | ChEBI | | Hyoscine | ChEBI | | Scopine (-)-tropate | ChEBI | | Transderm-scop | ChEBI | | (1S,3S,5R,6R,7S)-6,7-Epoxytropan-3-yl (2S)-3-hydroxy-2-phenylpropanoic acid | Generator | | 6,7-Epoxytropine tropic acid | Generator | | 6-b,7-b-Epoxy-3-a-tropanyl S-(-)-tropate | Generator | | 6-b,7-b-Epoxy-3-a-tropanyl S-(-)-tropic acid | Generator | | 6-beta,7-beta-Epoxy-3-alpha-tropanyl S-(-)-tropic acid | Generator | | 6-Β,7-β-epoxy-3-α-tropanyl S-(-)-tropate | Generator | | 6-Β,7-β-epoxy-3-α-tropanyl S-(-)-tropic acid | Generator | | a-(Hydroxymethyl)benzeneacetate 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl ester | Generator | | a-(Hydroxymethyl)benzeneacetic acid 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl ester | Generator | | alpha-(Hydroxymethyl)benzeneacetate 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl ester | Generator | | Α-(hydroxymethyl)benzeneacetate 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl ester | Generator | | Α-(hydroxymethyl)benzeneacetic acid 9-methyl-3-oxa-9-azatricyclo(3.3.1.0(2.4))non-7-yl ester | Generator | | Scopine (-)-tropic acid | Generator | | (+)-Hyoscine | HMDB | | (+)-Scopolamine | HMDB | | (-)-Hyoscine hydrobromide | HMDB | | (-)-Scopolamine bromide | HMDB | | (-)-Scopolamine hydrobromide | HMDB | | Atrochin | HMDB | | Atroquin | HMDB | | Beldavrin | HMDB | | Buscopan | HMDB | | Epoxytropine tropate | HMDB | | Euscopol | HMDB | | Hydroscine hydrobromide | HMDB | | Hyosceine | HMDB | | Hyoscine bromide | HMDB | | Hyoscine hydrobromide | HMDB | | Hyoscyine hydrobromide | HMDB | | Hyosol | HMDB | | Hysco | HMDB | | Isopto hyoscine | HMDB | | Isoscopil | HMDB | | Kwells | HMDB | | L-Hyoscine hydrobromide | HMDB | | L-Scopolamine-hydrobromide | HMDB | | Methscopolamine bromide | HMDB | | Oscine | HMDB | | Pamine | HMDB | | S-(-)-Tropate | HMDB | | Scop | HMDB | | Scopamin | HMDB | | Scopine tropate | HMDB | | Scopolamine bromide | HMDB | | Scopolamine hydrobromide | HMDB | | Scopolaminium bromide | HMDB | | Scopolammonium bromide | HMDB | | SEE | HMDB | | Tranaxine | HMDB | | Norhyoscine | HMDB | | 6beta,7beta-Epoxy-3alpha-tropanyl S-(-)-tropate | PhytoBank | | 6β,7β-Epoxy-3α-tropanyl S-(-)-tropate | PhytoBank | | 9-Methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7-ol (-)-tropate | PhytoBank | | l-Scopolamine | PhytoBank | | (-)-Atropine | PhytoBank | | (-)-Hyoscyamine | PhytoBank | | (S)-(-)-Hyoscyamine | PhytoBank | | (S)-Atropine | PhytoBank | | 1alphaH,5alphaH-Tropan-3alpha-yl (-)-tropate | PhytoBank | | 1αH,5αH-Tropan-3α-yl (-)-tropate | PhytoBank | | Cystospaz | PhytoBank | | Daturine | PhytoBank | | Duboisine | PhytoBank | | Hyoscyamine | PhytoBank | | L-Hyoscyamin | PhytoBank | | l-Hyoscyamine | PhytoBank | | l-Atropine | PhytoBank | | l-Tropine tropate | PhytoBank | | (±)-Atropine | PhytoBank | | (±)-Hyoscyamine | PhytoBank | | Atropin | PhytoBank | | Atropine | PhytoBank | | Atropinum sulfuricum | PhytoBank | | Atropinum sulphuricum | PhytoBank | | dl-Hyoscyamine | PhytoBank | | Tropine (±)-tropate | PhytoBank | | Tropine tropate | PhytoBank | | dl-Tropyl tropate | PhytoBank |
|
|---|
| Chemical Formula | C17H21NO4 |
|---|
| Average Molecular Weight | 303.3529 |
|---|
| Monoisotopic Molecular Weight | 303.147058165 |
|---|
| IUPAC Name | (1R,2R,4S,5S,7S)-9-methyl-3-oxa-9-azatricyclo[3.3.1.0^{2,4}]nonan-7-yl (2S)-3-hydroxy-2-phenylpropanoate |
|---|
| Traditional Name | (1R,2R,4S,5S,7S)-9-methyl-3-oxa-9-azatricyclo[3.3.1.0^{2,4}]nonan-7-yl (2S)-3-hydroxy-2-phenylpropanoate |
|---|
| CAS Registry Number | 51-34-3 |
|---|
| SMILES | CN1[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3/t11-,12-,13-,14+,15-,16+/m1/s1 |
|---|
| InChI Key | STECJAGHUSJQJN-FWXGHANASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta-hydroxy acid
- Monocyclic benzene moiety
- Morpholine
- Oxazinane
- Piperidine
- N-alkylpyrrolidine
- Benzenoid
- Pyrrolidine
- Amino acid or derivatives
- Carboxylic acid ester
- Tertiary amine
- Tertiary aliphatic amine
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid derivative
- Dialkyl ether
- Oxirane
- Ether
- Monocarboxylic acid or derivatives
- Primary alcohol
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Amine
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-0f7c-7900000000-28f924e821203772c1ad | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-4910000000-24c90e50ab88ad57e82c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0006-4900000000-596180a39c76ec0fe1ae | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0udi-0009000000-9733effdc3262b114ed6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0udi-0809000000-a686e4c768aedb57cf8e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-000i-1901000000-f66f538c7db471e86fd5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-000i-3900000000-5eedeba4bedea55de9ad | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0udr-7900000000-1309364bbca3810a6afa | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT , positive | splash10-000i-0900000000-1c1d2f52fb7a5d745e1e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0udi-9700000000-80887cb9e3f0d05c8bf5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0udi-9800000000-80887cb9e3f0d05c8bf5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-0udi-6900000000-010e6e0ca9f0d9f3618b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0f79-4900000000-a0695c5add6db92de8b2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-000i-2900000000-887570e409db5eca481c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0f79-4900000000-5e1feadfe643a530d956 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-000i-2900000000-9556767e32b410cf8d73 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-0udi-0309000000-fda2d2422fa0a3b4172e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0k9i-0903000000-cc938997b1c67e020c14 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-0955000000-64498c50c4e2afb15c32 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1930000000-5d768d7bc9f10614fd97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0lxt-4900000000-f39cfd7374383b7b1aaf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0549000000-2102ba2525a572e05e74 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uk9-1952000000-23caf7102d633b0031b2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0hr0-4900000000-854ad2f78678cc2d4db3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6t-0904000000-0366209ad3f767f88736 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f79-1900000000-bd791764fd2502fdc39d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gb9-2910000000-c7c4ffc22c1425ab3dbd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0019000000-cc4ab81a673ee149c93e | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, CD3OD, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CD3OD, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|