| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:08:54 UTC |
|---|
| Update Date | 2020-04-22 15:13:37 UTC |
|---|
| BMDB ID | BMDB0004256 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 7-Hydroxy-6-methyl-8-ribityl lumazine |
|---|
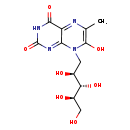
| Description | 7-Hydroxy-6-methyl-8-ribityl lumazine, also known as 7-hydroxy-6-methyl-8-ribityl lumazine or RL-6-me-7-OH, belongs to the class of organic compounds known as pteridines and derivatives. These are polycyclic aromatic compounds containing a pteridine moiety, which consists of a pyrimidine fused to a pyrazine ring to form pyrimido(4,5-b)pyrazine. 7-Hydroxy-6-methyl-8-ribityl lumazine is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). In cattle, 7-hydroxy-6-methyl-8-ribityl lumazine is involved in the metabolic pathway called the riboflavin metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Deoxy-1-(3,4-dihydro-7-hydroxy-6-methyl-2,4-dioxo-8(2H)-pteridinyl)-D-ribitol | ChEBI | | 7-Hydroxy-6-methyl-8-D-ribityllumazine | ChEBI | | Masuda's compound V | ChEBI | | RL-6-Me-7-OH | ChEBI | | 7-Oxolumazine | Kegg | | 6-Methyl-7-oxo-8-ribityllumazine | Kegg | | CRM | HMDB |
|
|---|
| Chemical Formula | C12H16N4O7 |
|---|
| Average Molecular Weight | 328.278 |
|---|
| Monoisotopic Molecular Weight | 328.101898886 |
|---|
| IUPAC Name | 7-hydroxy-6-methyl-8-[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]-2,3,4,8-tetrahydropteridine-2,4-dione |
|---|
| Traditional Name | 7-hydroxy-6-methyl-8-[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]-3H-pteridine-2,4-dione |
|---|
| CAS Registry Number | 2184-54-5 |
|---|
| SMILES | CC1=C(O)N(C[C@H](O)[C@H](O)[C@H](O)CO)C2=NC(=O)NC(=O)C2=N1 |
|---|
| InChI Identifier | InChI=1S/C12H16N4O7/c1-4-11(22)16(2-5(18)8(20)6(19)3-17)9-7(13-4)10(21)15-12(23)14-9/h5-6,8,17-20,22H,2-3H2,1H3,(H,15,21,23)/t5-,6+,8-/m0/s1 |
|---|
| InChI Key | COXMGTTXHPRZBO-BBVRLYRLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pteridines and derivatives. These are polycyclic aromatic compounds containing a pteridine moiety, which consists of a pyrimidine fused to a pyrazine ring to form pyrimido(4,5-b)pyrazine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Pteridines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pteridine
- Pyrimidone
- Pyrazine
- Pyrimidine
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Secondary alcohol
- Polyol
- Azacycle
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary alcohol
- Alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | -4.193 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-9031000000-31345cb74c25ba581782 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-00di-1011119000-4e9da45e71f2d8ecf3cb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_4_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_4_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_5_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("7-Hydroxy-6-methyl-8-ribityl lumazine,3TBDMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-0049000000-c96549d32e50830eb68e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-08fr-5291000000-f24a253e13e58999151e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08i0-2910000000-cf0790ca9d8484d3a659 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0089-3090000000-ae929200294d52194b44 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9100000000-b54d6bfe5deeef630a9c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9800000000-7eba918d7919fe2c040c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0409000000-29d1140e0df4e7517be1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1093000000-92e87bd1573b8696c107 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0790000000-e5d913a0bdfaa904384d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-056r-0069000000-9efece673f44ae85fa67 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066v-2390000000-e6f235ac58abf1514d1d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-5910000000-0fd8a00cea868010c4bd | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|