| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:10:44 UTC |
|---|
| Update Date | 2020-04-22 15:14:11 UTC |
|---|
| BMDB ID | BMDB0004822 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Methyl bisnorbiotinyl ketone |
|---|
| Description | Methyl bisnorbiotinyl ketone belongs to the class of organic compounds known as thienoimidazolidines. These are heterocyclic compounds containing a thiophene ring fused to an imidazolidine ring. Thiophene is 5-membered ring consisting of four carbon atoms and one sulfur atom. Imidazolidine is 5-membered saturated ring of three carbon atoms, and two nitrogen centers at the 1- and 3-positions. Based on a literature review a significant number of articles have been published on Methyl bisnorbiotinyl ketone. |
|---|
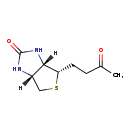
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| [3AS-(3aa,4b,6aa)]-tetrahydro-4-(3-oxobutyl)-1H-thieno[3,4-D]imidazol-2(3H)-one | HMDB | | Bisnorbiotin methyl ketone | MeSH, HMDB |
|
|---|
| Chemical Formula | C9H14N2O2S |
|---|
| Average Molecular Weight | 214.285 |
|---|
| Monoisotopic Molecular Weight | 214.077598392 |
|---|
| IUPAC Name | (3aS,4S,6aR)-4-(3-oxobutyl)-hexahydro-1H-thieno[3,4-d]imidazolidin-2-one |
|---|
| Traditional Name | (3aS,4S,6aR)-4-(3-oxobutyl)-hexahydrothieno[3,4-d]imidazolidin-2-one |
|---|
| CAS Registry Number | 35638-35-8 |
|---|
| SMILES | [H][C@]12CS[C@@H](CCC(C)=O)[C@@]1([H])NC(=O)N2 |
|---|
| InChI Identifier | InChI=1S/C9H14N2O2S/c1-5(12)2-3-7-8-6(4-14-7)10-9(13)11-8/h6-8H,2-4H2,1H3,(H2,10,11,13)/t6-,7-,8-/m0/s1 |
|---|
| InChI Key | ITHUGYGPPSVHRH-FXQIFTODSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thienoimidazolidines. These are heterocyclic compounds containing a thiophene ring fused to an imidazolidine ring. Thiophene is 5-membered ring consisting of four carbon atoms and one sulfur atom. Imidazolidine is 5-membered saturated ring of three carbon atoms, and two nitrogen centers at the 1- and 3-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Thienoimidazolidines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Thienoimidazolidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thienoimidazolidine
- Imidazolidinone
- Imidazolidine
- Thiolane
- Thiophene
- Ketone
- Carbonic acid derivative
- Urea
- Thioether
- Dialkylthioether
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9500000000-b5dcc79e344bbe89b7e4 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014j-0970000000-21fdb57ab3577709d301 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-1920000000-c111d995b85ccbc31a82 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pc3-9800000000-714044161a3db1349e15 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1690000000-6da85b9dcf8a112e196d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-07ou-8920000000-4702c671e9e2014d2e9c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-cf68c73d5fe1400174b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-359bd2c7030f5470cb12 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-1590000000-30b94177843601b0aa30 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000b-9600000000-089432c95b74f38f7652 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-df9f468a8a556ceb5254 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-3980000000-eed66ce0b19d7754de8f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-aa6f041cfab69df4193f | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|