| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:14:53 UTC |
|---|
| Update Date | 2020-05-11 20:49:34 UTC |
|---|
| BMDB ID | BMDB0005034 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Topiramate |
|---|
| Description | Topiramate, also known as topamax or MCN-4853, belongs to the class of organic compounds known as dioxolopyrans. Dioxolopyrans are compounds containing a dioxolopyran moiety, which consists of a dioxole ring fused to a pyran ring. Topiramate is an extremely weak basic (essentially neutral) compound (based on its pKa). Topiramate is a potentially toxic compound. |
|---|
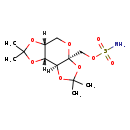
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,3:4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate | ChEBI | | 2,3:4,5-Di-O-isopropylidene-beta-D-fructopyranose sulfamate | ChEBI | | MCN-4853 | ChEBI | | RWJ-17021 | ChEBI | | Tipiramate | ChEBI | | Tipiramato | ChEBI | | Topamax | ChEBI | | Topiramato | ChEBI | | Topiramatum | ChEBI | | TPM | ChEBI | | Trokendi XR | Kegg | | 2,3:4,5-Bis-O-(1-methylethylidene)-b-D-fructopyranose sulfamate | Generator | | 2,3:4,5-Bis-O-(1-methylethylidene)-b-D-fructopyranose sulfamic acid | Generator | | 2,3:4,5-Bis-O-(1-methylethylidene)-b-D-fructopyranose sulphamate | Generator | | 2,3:4,5-Bis-O-(1-methylethylidene)-b-D-fructopyranose sulphamic acid | Generator | | 2,3:4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamic acid | Generator | | 2,3:4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulphamate | Generator | | 2,3:4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulphamic acid | Generator | | 2,3:4,5-Bis-O-(1-methylethylidene)-β-D-fructopyranose sulfamate | Generator | | 2,3:4,5-Bis-O-(1-methylethylidene)-β-D-fructopyranose sulfamic acid | Generator | | 2,3:4,5-Bis-O-(1-methylethylidene)-β-D-fructopyranose sulphamate | Generator | | 2,3:4,5-Bis-O-(1-methylethylidene)-β-D-fructopyranose sulphamic acid | Generator | | 2,3:4,5-Di-O-isopropylidene-b-D-fructopyranose sulfamate | Generator | | 2,3:4,5-Di-O-isopropylidene-b-D-fructopyranose sulfamic acid | Generator | | 2,3:4,5-Di-O-isopropylidene-b-D-fructopyranose sulphamate | Generator | | 2,3:4,5-Di-O-isopropylidene-b-D-fructopyranose sulphamic acid | Generator | | 2,3:4,5-Di-O-isopropylidene-beta-D-fructopyranose sulfamic acid | Generator | | 2,3:4,5-Di-O-isopropylidene-beta-D-fructopyranose sulphamate | Generator | | 2,3:4,5-Di-O-isopropylidene-beta-D-fructopyranose sulphamic acid | Generator | | 2,3:4,5-Di-O-isopropylidene-β-D-fructopyranose sulfamate | Generator | | 2,3:4,5-Di-O-isopropylidene-β-D-fructopyranose sulfamic acid | Generator | | 2,3:4,5-Di-O-isopropylidene-β-D-fructopyranose sulphamate | Generator | | 2,3:4,5-Di-O-isopropylidene-β-D-fructopyranose sulphamic acid | Generator | | Tipiramic acid | Generator | | Topiramic acid | Generator | | Epitomax | HMDB | | Topamax sprinkle | HMDB | | Topomax | HMDB | | 2,3-4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate | HMDB |
|
|---|
| Chemical Formula | C12H21NO8S |
|---|
| Average Molecular Weight | 339.362 |
|---|
| Monoisotopic Molecular Weight | 339.098787343 |
|---|
| IUPAC Name | [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0²,⁶]dodecan-6-yl]methyl sulfamate |
|---|
| Traditional Name | topiramate |
|---|
| CAS Registry Number | 97240-79-4 |
|---|
| SMILES | [H][C@@]12CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@@]3([H])[C@]1([H])OC(C)(C)O2 |
|---|
| InChI Identifier | InChI=1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1 |
|---|
| InChI Key | KJADKKWYZYXHBB-XBWDGYHZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dioxolopyrans. Dioxolopyrans are compounds containing a dioxolopyran moiety, which consists of a dioxole ring fused to a pyran ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dioxolopyrans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Dioxolopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dioxolopyran
- Ketal
- Oxane
- Monosaccharide
- Organic sulfuric acid or derivatives
- Meta-dioxolane
- Oxacycle
- Acetal
- Organic nitrogen compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 125 - 126 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-003r-5893000000-57939ef42b569234ad29 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-03di-0239000000-994a7b97e101c455a792 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-01x0-1970000000-f0e80f5cc5d6fc2b6d38 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-01q9-2960000000-a11a150bcada5055d2e9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-014i-0090000000-c1fa3d6ff15890fc1418 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , positive | splash10-00di-0059000000-a6337903cab01edce60e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-03di-0239000000-994a7b97e101c455a792 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-01x0-1970000000-f0e80f5cc5d6fc2b6d38 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Negative | splash10-004i-9000000000-a8df5a6ea132d69b9489 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-004i-9000000000-f76d8f2283f18dfa20be | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-004i-9000000000-316320612e65b9d317cb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-004i-9000000000-63d6b2cbde40b2bcd639 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-004i-9003000000-0b8ee634478a6f2f0eee | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-000i-0009000000-086098dbff51e692c04f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0c0r-9850000000-ae199550ba647589f088 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-004i-9000000000-eab734dd0ed786cbba6d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-1029000000-37869fd8dcd3ec0f4cc1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01po-4295000000-7cdbdca2d1d67b21879d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03fr-9810000000-9b5c3fb12c27e09cf450 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002r-4239000000-e56f7b1fecd3064e8783 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004j-9164000000-892445ea4dcd3bf9420d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9100000000-7c64b90d0daae2c6e5b8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-9a74f9cc10019d27995c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0149000000-093e852afab8097cfa0f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dm-8790000000-0a33128bbe614c27cdf7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0019000000-345311bd602424b95a49 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|