| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:15:00 UTC |
|---|
| Update Date | 2020-04-22 15:15:13 UTC |
|---|
| BMDB ID | BMDB0005045 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 15(S)-Hydroxyeicosatrienoic acid |
|---|
| Description | 15(S)-Hydroxyeicosatrienoic acid, also known as 15-hetre or 15S-hydroxy-8Z,11Z,13E-eicosatrienoate, belongs to the class of organic compounds known as hydroxyeicosatrienoic acids. These are eicosanoic acids with an attached hydroxyl group and three CC double bonds. Based on a literature review a significant number of articles have been published on 15(S)-Hydroxyeicosatrienoic acid. |
|---|
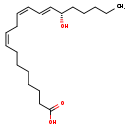
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (8Z,11Z,13E,15S)-15-Hydroxyicosatrienoic acid | ChEBI | | 15-HETrE | ChEBI | | 15-Hydroxy-(8Z,11Z,13E)-eicosatrienoic acid | ChEBI | | 15-Hydroxy-cis,cis,trans-8,11,13-eicosatrienoic acid | ChEBI | | 15-Hydroxyeicosatrienoic acid | ChEBI | | 15S-HETrE | ChEBI | | 15S-Hydroxy-8Z,11Z,13E-eicosatrienoic acid | ChEBI | | (8Z,11Z,13E,15S)-15-Hydroxyicosatrienoate | Generator | | 15-Hydroxy-(8Z,11Z,13E)-eicosatrienoate | Generator | | 15-Hydroxy-cis,cis,trans-8,11,13-eicosatrienoate | Generator | | 15-Hydroxyeicosatrienoate | Generator | | 15S-Hydroxy-8Z,11Z,13E-eicosatrienoate | Generator | | 15(S)-Hydroxyeicosatrienoate | Generator |
|
|---|
| Chemical Formula | C20H34O3 |
|---|
| Average Molecular Weight | 322.4822 |
|---|
| Monoisotopic Molecular Weight | 322.250794954 |
|---|
| IUPAC Name | (8Z,11Z,13E,15S)-15-hydroxyicosa-8,11,13-trienoic acid |
|---|
| Traditional Name | 6-deoxy-L-sorbose |
|---|
| CAS Registry Number | 13-16-1 |
|---|
| SMILES | CCCCC[C@H](O)\C=C\C=C/C\C=C/CCCCCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H34O3/c1-2-3-13-16-19(21)17-14-11-9-7-5-4-6-8-10-12-15-18-20(22)23/h4-5,9,11,14,17,19,21H,2-3,6-8,10,12-13,15-16,18H2,1H3,(H,22,23)/b5-4-,11-9-,17-14+/t19-/m0/s1 |
|---|
| InChI Key | IUKXMNDGTWTNTP-OAHXIXLCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxyeicosatrienoic acids. These are eicosanoic acids with an attached hydroxyl group and three CC double bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Hydroxyeicosatrienoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyeicosatrienoic acid
- Long-chain fatty acid
- Hydroxy fatty acid
- Fatty acid
- Unsaturated fatty acid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxide
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0pi0-6592000000-9dc0f3f0bbc2de8a434d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-004i-9332200000-cf5f429c3867c843e3f2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0059000000-a80d73087c4ff885f762 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-4393000000-64b119d876007616dced | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03kc-9630000000-2bff72917aba29b0568d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0019000000-4c24ada22b9690dcb2fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fk9-2059000000-6a462b1cbfb1f2633ec2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9130000000-973ff7b5957c59e99327 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fk9-0009000000-57568fa8a4085a5e528c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uk9-1249000000-c0ee7a1e26de1f84a01c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9140000000-a469f1131b8f8929c25e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0459000000-12a2a03e81c41e9d9b57 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-1953000000-ce7b9656736b3429c15a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-067i-9600000000-dee10fd76962f5b5aae8 | View in MoNA |
|---|
|
|---|