| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:15:38 UTC |
|---|
| Update Date | 2020-05-21 16:27:36 UTC |
|---|
| BMDB ID | BMDB0005356 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | TG(16:0/16:0/16:0)[iso] |
|---|
| Description | TG(16:0/16:0/16:0), also known as tripalmitoylglycerol or glyceryl tripalmitate, belongs to the class of organic compounds known as triacylglycerols. These are glycerides consisting of three fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Thus, TG(16:0/16:0/16:0) is considered to be a triradylglycerol lipid molecule. TG(16:0/16:0/16:0) is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. TG(16:0/16:0/16:0) exists in all eukaryotes, ranging from yeast to humans. In cattle, TG(16:0/16:0/16:0) is involved in the metabolic pathway called the glycerolipid metabolism pathway. |
|---|
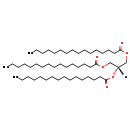
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2,3-Propanetriol trihexadecanoate | ChEBI | | 1,2,3-Propanetriyl trihexadecanoate | ChEBI | | 1,2,3-Trihexadecanoylglycerol | ChEBI | | Glycerin tripalmitate | ChEBI | | Glycerol tripalmitate | ChEBI | | Glyceryl trihexadecanoate | ChEBI | | Glyceryl tripalmitate | ChEBI | | Hexadecanoic acid, 1,2,3-propanetriyl ester | ChEBI | | Palmitic acid triglycerin ester | ChEBI | | Palmitic triglyceride | ChEBI | | TG 16:0/16:0/16:0 | ChEBI | | Triglyceryl palmitate | ChEBI | | Trihexadecanoylglycerol | ChEBI | | Tripalmitoylglycerol | ChEBI | | 1,2,3-Propanetriol trihexadecanoic acid | Generator | | 1,2,3-Propanetriyl trihexadecanoic acid | Generator | | Glycerin tripalmitic acid | Generator | | Glycerol tripalmitic acid | Generator | | Glyceryl trihexadecanoic acid | Generator | | Glyceryl tripalmitic acid | Generator | | Hexadecanoate, 1,2,3-propanetriyl ester | Generator | | Palmitate triglycerin ester | Generator | | Triglyceryl palmitic acid | Generator | | Glycero-tripalmitate | MeSH | | Tripalmitoyl glycerol | MeSH | | Tripalmitylglycerol | MeSH | | 1-palmitoyl-2-palmitoyl-3-palmitoyl-glycerol | Lipid Annotator, HMDB | | TG(16:0/16:0/16:0) | Lipid Annotator, ChEBI | | TG(48:0) | Lipid Annotator, HMDB | | Triglyceride | Lipid Annotator, HMDB | | Tracylglycerol(48:0) | Lipid Annotator, HMDB | | TAG(16:0/16:0/16:0) | Lipid Annotator, HMDB | | Tracylglycerol(16:0/16:0/16:0) | Lipid Annotator, HMDB | | TAG(48:0) | Lipid Annotator, HMDB | | 1-hexadecanoyl-2-hexadecanoyl-3-hexadecanoyl-glycerol | Lipid Annotator, HMDB | | Triacylglycerol | Lipid Annotator, HMDB | | 1,2,3-Trihexadecanoyl-sn-glycerol | HMDB | | Barolub LCD | HMDB | | Dynasan 116 | HMDB | | Dynosan 114 | HMDB | | Spezialfett 116 | HMDB | | Triglyceride PPP | HMDB | | Tripalmitate | HMDB | | Tripalmitin | HMDB |
|
|---|
| Chemical Formula | C51H98O6 |
|---|
| Average Molecular Weight | 807.3202 |
|---|
| Monoisotopic Molecular Weight | 806.736340868 |
|---|
| IUPAC Name | 1,3-bis(hexadecanoyloxy)propan-2-yl hexadecanoate |
|---|
| Traditional Name | tripalmitin |
|---|
| CAS Registry Number | 555-44-2 |
|---|
| SMILES | [H]C(COC(=O)CCCCCCCCCCCCCCC)(COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C51H98O6/c1-4-7-10-13-16-19-22-25-28-31-34-37-40-43-49(52)55-46-48(57-51(54)45-42-39-36-33-30-27-24-21-18-15-12-9-6-3)47-56-50(53)44-41-38-35-32-29-26-23-20-17-14-11-8-5-2/h48H,4-47H2,1-3H3 |
|---|
| InChI Key | PVNIQBQSYATKKL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triacylglycerols. These are glycerides consisting of three fatty acid chains covalently bonded to a glycerol molecule through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerolipids |
|---|
| Sub Class | Triradylcglycerols |
|---|
| Direct Parent | Triacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triacyl-sn-glycerol
- Tricarboxylic acid or derivatives
- Fatty acid ester
- Fatty acyl
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udr-4266090000-d72def42ee8b15e7d8ab | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udi-5255090000-f2b69e4c01f94e964a8e | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udr-4266090000-d72def42ee8b15e7d8ab | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udi-5255090000-f2b69e4c01f94e964a8e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80) , Positive | splash10-0udr-4266090000-d72def42ee8b15e7d8ab | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80) , Positive | splash10-0udi-5255090000-08a84d0a82ce5839332e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (ACQUITY UPLC System, Waters) 30V, Positive | splash10-0udi-0000090000-b971f7be0932cc17d4fc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (ACQUITY UPLC System, Waters) 30V, Positive | splash10-0udi-0000090000-ba2f475d5deb56cbcb0f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000000090-2dcd9d1c90fb060e2d76 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0000000090-2dcd9d1c90fb060e2d76 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-0000090070-5e4488c43f4273c179dd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0070080190-e59a843e2ac9eb401541 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-053r-0090020000-1e6cc9453781ea6f2f0e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-1090010000-52dbd7332c4689b8becb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000000090-497b97a123d4ddbee56b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000000090-497b97a123d4ddbee56b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08fr-0090090090-3d1ee4df7694f2a67b63 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000000090-feacb9716f0cce2e39b9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0000000090-feacb9716f0cce2e39b9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-0000000090-feacb9716f0cce2e39b9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000000090-c9b5cf0ac5a5011ec584 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0000000090-c9b5cf0ac5a5011ec584 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-0010090070-13293a79e092976cb1e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-4130052390-48f5c7a747823dfa0644 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-059i-9150123400-dde2539447879b15fbf1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000l-4496013000-d20d940fe1d4244aa3e7 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 90 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|