| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:19:15 UTC |
|---|
| Update Date | 2020-06-04 20:42:36 UTC |
|---|
| BMDB ID | BMDB0005895 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
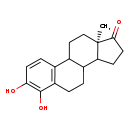
| Common Name | 4-Hydroxyestrone |
|---|
| Description | 4-Hydroxyestrone belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. Based on a literature review a significant number of articles have been published on 4-Hydroxyestrone. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,4-Dihydroxy-1,3,5(10)-estratrien-17-one | MeSH | | 1,3,5(10)-Estratriene-3,4-diol-17-one | HMDB | | 3,4-Dihydroxy-estra-1,3,5(10)-trien-17-one | HMDB | | 3,4-Dihydroxyestra-1,3,5(10)-trien-17-one | HMDB | | 4-Hydroxy catechol estrogen | HMDB | | 4OHEs | HMDB | | Estra-1,3,5(10)-triene-3,4-diol-17-one | HMDB | | 4-Hydroxyestrone | MeSH |
|
|---|
| Chemical Formula | C18H22O3 |
|---|
| Average Molecular Weight | 286.3655 |
|---|
| Monoisotopic Molecular Weight | 286.15689457 |
|---|
| IUPAC Name | (15R)-5,6-dihydroxy-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2(7),3,5-trien-14-one |
|---|
| Traditional Name | (15R)-5,6-dihydroxy-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-2(7),3,5-trien-14-one |
|---|
| CAS Registry Number | 3131-23-5 |
|---|
| SMILES | C[C@@]12CCC3C(CCC4=C3C=CC(O)=C4O)C1CCC2=O |
|---|
| InChI Identifier | InChI=1S/C18H22O3/c1-18-9-8-11-10-4-6-15(19)17(21)13(10)3-2-12(11)14(18)5-7-16(18)20/h4,6,11-12,14,19,21H,2-3,5,7-9H2,1H3/t11?,12?,14?,18-/m1/s1 |
|---|
| InChI Key | XQZVQQZZOVBNLU-KQWCVQSUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-hydroxysteroid
- 4-hydroxysteroid
- Hydroxysteroid
- 17-oxosteroid
- Oxosteroid
- Phenanthrene
- Tetralin
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Ketone
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0bvu-0980000000-41ba72b2ddfbd882f4c6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01b9-1009500000-d5f44360a885dcec716a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0190000000-c34b61a05cd2e3f49e72 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0890000000-37625f295d4a5ca9b26a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-9480000000-200c72b672bae479797a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-a7196dc023614e391397 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-b6c978d06836a11c4e6a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aou-1090000000-82830726aa968d395703 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-fcaf0435bbf678a207f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-e74df457a0a0026c8248 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06w9-0390000000-02e298c2aba8de880c76 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-0ebc482c8deef93e8af1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0390000000-2d8777d2312bf283a06a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01t9-0920000000-415199c8e432ed8b230b | View in MoNA |
|---|
|
|---|