| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:19:46 UTC |
|---|
| Update Date | 2020-04-22 15:16:43 UTC |
|---|
| BMDB ID | BMDB0006031 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 11-Ketoetiocholanolone |
|---|
| Description | 11-Ketoetiocholanolone belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. Based on a literature review a significant number of articles have been published on 11-Ketoetiocholanolone. |
|---|
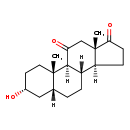
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3alpha-Hydroxy-5beta-androstane-11,17-dione | Kegg | | 3a-Hydroxy-5b-androstane-11,17-dione | Generator | | 3Α-hydroxy-5β-androstane-11,17-dione | Generator | | (3alpha,5beta)-3-Hydroxy-androstane-11,17-dione | HMDB | | 11-Oxoaetiocholanolone | HMDB | | 11-Oxoetiocholanolone | HMDB, MeSH | | 3-Hydroxyandrostane-11,17-dione | HMDB | | 3alpha-Hydroxy-11,17-dioxo-5beta-androstane | HMDB | | 5beta-Androstan-3alpha-ol-11,17-dione | HMDB | | 11-O-ETIO | MeSH, HMDB |
|
|---|
| Chemical Formula | C19H28O3 |
|---|
| Average Molecular Weight | 304.4238 |
|---|
| Monoisotopic Molecular Weight | 304.203844762 |
|---|
| IUPAC Name | (1S,2S,5R,7R,10S,11S,15S)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecane-14,17-dione |
|---|
| Traditional Name | 11-oxoetiocholanolone |
|---|
| CAS Registry Number | 739-27-5 |
|---|
| SMILES | [H][C@@]12CCC(=O)[C@@]1(C)CC(=O)[C@@]1([H])[C@@]2([H])CC[C@]2([H])C[C@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C19H28O3/c1-18-8-7-12(20)9-11(18)3-4-13-14-5-6-16(22)19(14,2)10-15(21)17(13)18/h11-14,17,20H,3-10H2,1-2H3/t11-,12-,13+,14+,17-,18+,19+/m1/s1 |
|---|
| InChI Key | IUNYGQONJQTULL-UKZLPJRTSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- 3-hydroxysteroid
- 3-alpha-hydroxysteroid
- Oxosteroid
- 17-oxosteroid
- 11-oxosteroid
- Hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0zfr-1922000000-53a646d987218b34c941 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0ffc-1940000000-c56c62d1b425b535ab14 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0zfr-1922000000-53a646d987218b34c941 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0ffc-1940000000-c56c62d1b425b535ab14 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ta-0390000000-f899506dd631386381fd | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-03l1-1359000000-c44ead28a84ab75143ba | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0094000000-45446caf2b170add176c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ap0-0191000000-6d4e843374d8d34e755a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4v-4390000000-f0f132ec0460e0271895 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0029000000-6d9c39501754e6495c03 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0069000000-e15b7ac311ca7d73dac5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05g3-2190000000-fd2a395c97f30477076c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0097000000-a5548652b47d12cf610e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bti-1491000000-b8de030ab96a9e9ab096 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014j-2910000000-eac837cca1cc49f3236d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-93986d59045cc7fce551 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0029000000-048ab11b637fc14885e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0wmm-4193000000-34031e37293525e81098 | View in MoNA |
|---|
|
|---|