| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:20:05 UTC |
|---|
| Update Date | 2020-04-22 15:16:49 UTC |
|---|
| BMDB ID | BMDB0006061 |
|---|
| Secondary Accession Numbers | - BMDB0095939
- BMDB06061
- BMDB95939
|
|---|
| Metabolite Identification |
|---|
| Common Name | 4-Hydroxyphenylacetylglutamic acid |
|---|
| Description | 4-Hydroxyphenylacetylglutamic acid belongs to the class of organic compounds known as glutamic acid and derivatives. Glutamic acid and derivatives are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Based on a literature review a significant number of articles have been published on 4-Hydroxyphenylacetylglutamic acid. |
|---|
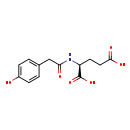
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Hydroxyphenylacetylglutamate | Generator | | p-Hydroxyphenylacetylglutamic acid | HMDB | | p-Hydroxyphenylacetylglutamate | HMDB | | (2S)-2-{[1-hydroxy-2-(4-hydroxyphenyl)ethylidene]amino}pentanedioate | HMDB | | 4-Hydroxyphenylacetylglutamic acid | HMDB |
|
|---|
| Chemical Formula | C13H15NO6 |
|---|
| Average Molecular Weight | 281.264 |
|---|
| Monoisotopic Molecular Weight | 281.089937207 |
|---|
| IUPAC Name | (2S)-2-[2-(4-hydroxyphenyl)acetamido]pentanedioic acid |
|---|
| Traditional Name | (2S)-2-[2-(4-hydroxyphenyl)acetamido]pentanedioic acid |
|---|
| CAS Registry Number | 1029120-37-3 |
|---|
| SMILES | OC(=O)CC[C@H](NC(=O)CC1=CC=C(O)C=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C13H15NO6/c15-9-3-1-8(2-4-9)7-11(16)14-10(13(19)20)5-6-12(17)18/h1-4,10,15H,5-7H2,(H,14,16)(H,17,18)(H,19,20)/t10-/m0/s1 |
|---|
| InChI Key | CYRKYXZJUIBBJX-JTQLQIEISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glutamic acid and derivatives. Glutamic acid and derivatives are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Glutamic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glutamic acid or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Benzenoid
- Dicarboxylic acid or derivatives
- Monocyclic benzene moiety
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid
- Carboximidic acid derivative
- Carboximidic acid
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03e9-0390000000-6d4c885b6c839ca27fac | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ka9-0930000000-db475847e7cc2c9f1ad1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kdi-7900000000-43df9129e7aae30c92fa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-3286bc974a189b026e7a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01qi-0980000000-d240fae32f133162a333 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zi3-5900000000-def93d56b16fcccc7678 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0930000000-282f2e31c8aed8e617d9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ufr-0900000000-a34f72a71240156e407e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9810000000-a1cd2469fd46adf2fa73 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001r-0690000000-44198a1f5da4d8d2d33d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zgr-0900000000-54bdb1c06149fff0c6ca | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-3900000000-ee69544db6ab6554cdca | View in MoNA |
|---|
|
|---|