| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:20:46 UTC |
|---|
| Update Date | 2020-04-22 15:17:02 UTC |
|---|
| BMDB ID | BMDB0006240 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Demethylated antipyrine |
|---|
| Description | Demethylated antipyrine belongs to the class of organic compounds known as phenylpyrazoles. Phenylpyrazoles are compounds containing a phenylpyrazole skeleton, which consists of a pyrazole bound to a phenyl group. Based on a literature review very few articles have been published on Demethylated antipyrine. |
|---|
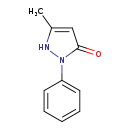
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Phenyl-3-methyl-1H-4,5-dihydropyrazol-5-one | HMDB | | 1-Phenyl-3-methyl-2-pyrazolin-5-one | HMDB | | 1-Phenyl-3-methyl-5-oxopyrazole | HMDB | | 1-Phenyl-3-methyl-5-pyrazolinone | HMDB | | 1-Phenyl-3-methyl-5-pyrazolone | HMDB | | 2,4-dihydro-5-Methyl-2-phenyl-3H-pyrazol-3-one | HMDB | | 3-Methyl-1-phenyl-1H-pyrazol-5-one | HMDB | | 3-Methyl-1-phenyl-2-pyrazolin-5-one | HMDB | | 3-Methyl-1-phenyl-2-pyrazoline-5-one | HMDB | | 3-Methyl-1-phenyl-4,5-dihydropyrazol-5-one | HMDB | | 3-Methyl-1-phenyl-4,5-dihydropyrazole-5-one | HMDB | | 3-Methyl-1-phenyl-5-pyrazolone | HMDB | | 3-Methyl-1-phenylpyrazol-5(4H)-one | HMDB | | 3-Methyl-1-phenylpyrazolin-5-one | HMDB | | 5-Methyl-2-phenyl-2H-pyrazol-3(4H)-one | HMDB | | 5-Methyl-2-phenylpyrazol-3-one | HMDB | | Edarabone | HMDB | | Edaravone | HMDB | | Methylphenylpyrazolone | HMDB | | Norantipyrine | HMDB | | Norphenazone | HMDB | | N-Demethylantipyrine, 14C-labeled | MeSH, HMDB | | N-Demethylantipyrine | MeSH, HMDB |
|
|---|
| Chemical Formula | C10H10N2O |
|---|
| Average Molecular Weight | 174.1992 |
|---|
| Monoisotopic Molecular Weight | 174.079312952 |
|---|
| IUPAC Name | 5-methyl-2-phenyl-2,3-dihydro-1H-pyrazol-3-one |
|---|
| Traditional Name | 5-methyl-2-phenyl-1H-pyrazol-3-one |
|---|
| CAS Registry Number | 89-25-8 |
|---|
| SMILES | CC1=CC(=O)N(N1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C10H10N2O/c1-8-7-10(13)12(11-8)9-5-3-2-4-6-9/h2-7,11H,1H3 |
|---|
| InChI Key | KZQYIMCESJLPQH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpyrazoles. Phenylpyrazoles are compounds containing a phenylpyrazole skeleton, which consists of a pyrazole bound to a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Pyrazoles |
|---|
| Direct Parent | Phenylpyrazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylpyrazole
- Monocyclic benzene moiety
- Pyrazolinone
- Benzenoid
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Azacycle
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 130 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-4900000000-e7fde844e072d094e7bf | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-ccca975ba1e597027ed5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0900000000-96da0ec122d8a8403486 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4r-9500000000-905aac7834f5ebcbec59 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-8e7cd9ae2599cec8be88 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-dbe1b04ba8ec0bb7b01a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-054k-6900000000-e3078766e74d43ea5dce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-99e013a33d61f5b2babb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0900000000-96887c840d6d9faeba92 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9100000000-a5215b963ef8a7e94117 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-6c988551b42e630dedc7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00xr-9600000000-0d26a32aab574f87a282 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9100000000-89d891f53ac7bcc51cdb | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|