| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:23:56 UTC |
|---|
| Update Date | 2020-04-22 15:18:02 UTC |
|---|
| BMDB ID | BMDB0006598 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
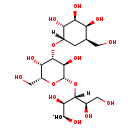
| Common Name | Isoglobotriaose |
|---|
| Description | Isoglobotriaose belongs to the class of organic compounds known as fatty acyl glycosides of mono- and disaccharides. Fatty acyl glycosides of mono- and disaccharides are compounds composed of a mono- or disaccharide moiety linked to one hydroxyl group of a fatty alcohol or of a phosphorylated alcohol (phosphoprenols), a hydroxy fatty acid or to one carboxyl group of a fatty acid (ester linkage) or to an amino alcohol. Based on a literature review a significant number of articles have been published on Isoglobotriaose. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| alpha-D-Galp-(1-4)-beta-D-galp-(1-4)-D-GLC | HMDB | | alpha-delta-Galp-(1-4)-beta-delta-galp-(1-4)-delta-GLC | HMDB | | Globoisotriaose | HMDB | | Isoglobotriglycosylceramide | HMDB | | O-alpha-D-Galactopyranosyl-(1->3)-O-beta-D-galactopyranosyl-(1->4)- D-glucose | HMDB | | O-alpha-delta-Galactopyranosyl-(1->3)-O-beta-delta-galactopyranosyl-(1->4)- D-glucose | HMDB |
|
|---|
| Chemical Formula | C19H34O15 |
|---|
| Average Molecular Weight | 502.4643 |
|---|
| Monoisotopic Molecular Weight | 502.189770418 |
|---|
| IUPAC Name | (2R,3R,4R,5R)-4-{[(2S,3R,4S,5S,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-{[(1S,2R,3S,4S,5R)-2,3,4-trihydroxy-5-(hydroxymethyl)cyclohexyl]oxy}oxan-2-yl]oxy}-2,3,5,6-tetrahydroxyhexanal |
|---|
| Traditional Name | isoglobotriaose |
|---|
| CAS Registry Number | 41744-59-6 |
|---|
| SMILES | [H][C@@](O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O[C@@]2([H])C[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)[C@H]1O)([C@H](O)CO)[C@H](O)[C@@H](O)C=O |
|---|
| InChI Identifier | InChI=1S/C19H34O15/c20-2-6-1-9(13(28)15(30)11(6)26)32-18-14(29)10(5-23)33-19(16(18)31)34-17(8(25)4-22)12(27)7(24)3-21/h3,6-20,22-31H,1-2,4-5H2/t6-,7+,8-,9+,10-,11+,12-,13+,14+,15+,16-,17-,18+,19+/m1/s1 |
|---|
| InChI Key | DRRWNKCTTZUPPM-FBSSLUAZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty acyl glycosides of mono- and disaccharides. Fatty acyl glycosides of mono- and disaccharides are compounds composed of a mono- or disaccharide moiety linked to one hydroxyl group of a fatty alcohol or of a phosphorylated alcohol (phosphoprenols), a hydroxy fatty acid or to one carboxyl group of a fatty acid (ester linkage) or to an amino alcohol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl glycosides |
|---|
| Direct Parent | Fatty acyl glycosides of mono- and disaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acyl glycoside of mono- or disaccharide
- Alkyl glycoside
- Disaccharide
- Glycosyl compound
- O-glycosyl compound
- Fatty alcohol
- Cyclohexanol
- Cyclitol or derivatives
- Beta-hydroxy aldehyde
- Oxane
- Cyclic alcohol
- Alpha-hydroxyaldehyde
- Secondary alcohol
- Polyol
- Ether
- Acetal
- Dialkyl ether
- Organoheterocyclic compound
- Oxacycle
- Primary alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aldehyde
- Alcohol
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03kl-5021900000-a07c227e99ec0149e48d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-003r-7326039000-967fd9fac550580b2342 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_21) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_22) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_86) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Isoglobotriaose,3TBDMS,#2" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q0-0700920000-d71f41a2022ffc4310cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-5901300000-7be09198d1b9a03e7297 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-7911000000-9361e68cf9181ca5a282 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0gwu-3101910000-b0182de80df14a123283 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004r-6906800000-60f7044f48ff68c78601 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004j-3900000000-5cd2e50225cb4d6404ae | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-02mi-1905720000-5d4825fed87b18a7bbd9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-6912100000-e8ce0b55191863ca8227 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03mj-9821000000-9ad90f8b602eeb3f976f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udl-4300950000-203283bd484f76f05efb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-6202910000-0381a53987a5a098b889 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9200000000-2e3df2a7876b181ab44e | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|