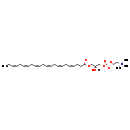| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:05:56 UTC |
|---|
| Update Date | 2020-05-11 19:11:25 UTC |
|---|
| BMDB ID | BMDB0010404 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | LysoPC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)) |
|---|
| Description | LysoPC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0) is a lysophospholipid (LyP). It is a monoglycerophospholipid in which a phosphorylcholine moiety occupies a glycerol substitution site. LysoPC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0), in particular, consists of one 4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoyl chain. Lysophosphatidylcholines can have different combinations of fatty acids of varying lengths and saturation attached at the C-1 (sn-1) position. Fatty acids containing 16, 18 and 20 carbons are the most common. LysoPC(20:3(5Z,8Z,11Z)), in particular, consists of one chain of mead acid at the C-1 position. The mead acid moiety is derived from fish oils, liver and kidney. Lysophosphatidylcholine is found in small amounts in most tissues. It is formed by hydrolysis of phosphatidylcholine by the enzyme phospholipase A2, as part of the de-acylation/re-acylation cycle that controls its overall molecular species composition. It can also be formed inadvertently during extraction of lipids from tissues if the phospholipase is activated by careless handling. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Docosahexaenoyl-glycero-3-phosphocholine | ChEBI | | LPC 22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0 | ChEBI | | LPC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0) | ChEBI | | Lyso(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0) | ChEBI | | LysoPC 22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0 | ChEBI | | Lysophosphatidylcholine(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0) | ChEBI | | LPC(22:6) | HMDB | | LPC(22:6/0:0) | HMDB | | LPC(22:6n3/0:0) | HMDB | | LPC(22:6W3/0:0) | HMDB | | LyPC(22:6) | HMDB | | LyPC(22:6/0:0) | HMDB | | LyPC(22:6n3/0:0) | HMDB | | LyPC(22:6W3/0:0) | HMDB | | LysoPC(22:6) | HMDB | | LysoPC(22:6/0:0) | HMDB | | LysoPC(22:6n3/0:0) | HMDB | | LysoPC(22:6W3/0:0) | HMDB | | Lysophosphatidylcholine(22:6) | HMDB | | Lysophosphatidylcholine(22:6/0:0) | HMDB | | Lysophosphatidylcholine(22:6n3/0:0) | HMDB | | Lysophosphatidylcholine(22:6W3/0:0) | HMDB | | 1-(4Z,7Z,10Z,13Z,16Z,19Z-Docosahexaenoyl)-glycero-3-phosphocholine | HMDB | | LysoPC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | HMDB | | 1-Docosahexaenoylglycerophosphocholine | HMDB | | 1-Docosahexaenoyl-GPC | HMDB | | 1-Docosahexaenoyl-lysophosphatidylcholine | HMDB | | 1-Docosahexaenoyl-sn-glycero-3-phosphocholine | HMDB | | DHA lysophosphatidylcholine | HMDB | | GPC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | HMDB | | GPC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0) | HMDB | | GPC(22:6) | HMDB | | GPC(22:6n3) | HMDB | | GPC(22:6n3/0:0) | HMDB | | GPC(22:6W3) | HMDB | | GPC(22:6W3/0:0) | HMDB | | LPC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | HMDB | | LPC(22:6n3) | HMDB | | LPC(22:6W3) | HMDB | | LysoPC(22:6n3) | HMDB | | LysoPC(22:6W3) | HMDB | | Lysophosphatidylcholine(22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | HMDB | | Lysophosphatidylcholine(22:6n3) | HMDB | | Lysophosphatidylcholine(22:6W3) | HMDB | | LysoPC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0) | HMDB |
|
|---|
| Chemical Formula | C30H50NO7P |
|---|
| Average Molecular Weight | 567.6943 |
|---|
| Monoisotopic Molecular Weight | 567.332489471 |
|---|
| IUPAC Name | (2-{[(2R)-3-[(4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoyloxy]-2-hydroxypropyl phosphono]oxy}ethyl)trimethylazanium |
|---|
| Traditional Name | (2-{[(2R)-3-[(4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoyloxy]-2-hydroxypropyl phosphono]oxy}ethyl)trimethylazanium |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@](O)(COC(=O)CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CC)COP([O-])(=O)OCC[N+](C)(C)C |
|---|
| InChI Identifier | InChI=1S/C30H50NO7P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23-24-30(33)36-27-29(32)28-38-39(34,35)37-26-25-31(2,3)4/h6-7,9-10,12-13,15-16,18-19,21-22,29,32H,5,8,11,14,17,20,23-28H2,1-4H3/b7-6-,10-9-,13-12-,16-15-,19-18-,22-21-/t29-/m1/s1 |
|---|
| InChI Key | LSOWKZULVQWMLY-APPDJCNMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-acyl-sn-glycero-3-phosphocholines. These are glycerophosphocholines in which the glycerol is esterified with a fatty acid at O-1 position, and linked at position 3 to a phosphocholine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphocholines |
|---|
| Direct Parent | 1-acyl-sn-glycero-3-phosphocholines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-acyl-sn-glycero-3-phosphocholine
- Phosphocholine
- Fatty acid ester
- Dialkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Secondary alcohol
- Carboxylic acid ester
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Alcohol
- Organic oxygen compound
- Organopnictogen compound
- Carbonyl group
- Organic salt
- Amine
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Intracellular membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0230-9433002000-b5fd41e28ea4799a79c2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("LysoPC(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/0:0),1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0000090000-e8bef32951c9eda16960 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01i0-0001090000-abe804b0d09d64c91508 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0frl-0309040000-3a407c5e1234e91b87af | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000090000-2b92165957071e6b3cb0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016r-1006190000-af60ed4bf75afc22985d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0109000000-2c24525447e59647ca0f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000090000-e0399259b48327bfbf18 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001l-0000090000-ba3d2d750970a47570b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pcr-1601790000-94e057a242a4c7624eb1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000039000-6a99405facca5a64946f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fb9-0009016000-ddefb625d8a0fb8adaa6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0039011000-dd78aa05e305d8717a4d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gb9-0000090000-67d3c276526724465aa3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00lr-0900060000-263d51168fd28cd1c380 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ue9-0910040000-ede15eec0297df860927 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|