| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-10-03 18:09:29 UTC |
|---|
| Update Date | 2020-05-21 16:28:08 UTC |
|---|
| BMDB ID | BMDB0010571 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
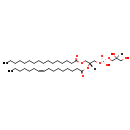
| Common Name | PG(16:0/16:1(9Z)) |
|---|
| Description | PG(16:0/16:1(9Z)), also known as PG(32:1) or GPG(16:0/16:1), belongs to the class of organic compounds known as phosphatidylglycerols. These are glycerophosphoglycerols in which two fatty acids are bonded to the 1-glycerol moiety through ester linkages. As is the case with diacylglycerols, phosphatidylglycerols can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. Thus, PG(16:0/16:1(9Z)) is considered to be a glycerophosphoglycerol lipid molecule. PG(16:0/16:1(9Z)) is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. PG(16:0/16:1(9Z)) exists in all living species, ranging from bacteria to humans. PG(16:0/16:1(9Z)) participates in a number of enzymatic reactions, within cattle. In particular, PG(16:0/16:1(9Z)) can be biosynthesized from PGP(16:0/16:1(9Z)); which is catalyzed by the enzyme phosphatidylglycerophosphatase and protein-tyrosine phosphatase 1. In addition, PG(16:0/16:1(9Z)) and CDP-DG(16:0/16:1(9Z)) can be converted into CL(16:0/16:1(9Z)/16:0/16:1(9Z)) and cytidine monophosphate through its interaction with the enzyme cardiolipin synthase. In cattle, PG(16:0/16:1(9Z)) is involved in the metabolic pathway called cardiolipin biosynthesis CL(16:0/16:1(9Z)/16:0/16:1(9Z)) pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| PG(32:1) | Lipid Annotator, HMDB | | PG(16:0/16:1(9Z)) | Lipid Annotator | | 1-palmitoyl-2-palmitoleoyl-sn-glycero-3-phosphoglycerol | Lipid Annotator, HMDB | | GPG(32:1) | Lipid Annotator, HMDB | | Phosphatidylglycerol(16:0/16:1) | Lipid Annotator, HMDB | | Phosphatidylglycerol(32:1) | Lipid Annotator, HMDB | | 1-hexadecanoyl-2-(9Z-hexadecenoyl)-sn-glycero-3-phospho-(1'-glycerol) | Lipid Annotator, HMDB | | GPG(16:0/16:1) | Lipid Annotator, HMDB | | 1-hexadecanoyl-2-(9Z-hexadecenoyl)-sn-glycero-3-phosphoglycerol | Lipid Annotator, HMDB | | PG(16:0/16:1) | Lipid Annotator, HMDB |
|
|---|
| Chemical Formula | C38H73O10P |
|---|
| Average Molecular Weight | 720.954 |
|---|
| Monoisotopic Molecular Weight | 720.494135068 |
|---|
| IUPAC Name | [(2S)-2,3-dihydroxypropoxy][(2R)-2-[(9Z)-hexadec-9-enoyloxy]-3-(hexadecanoyloxy)propoxy]phosphinic acid |
|---|
| Traditional Name | (2S)-2,3-dihydroxypropoxy((2R)-2-[(9Z)-hexadec-9-enoyloxy]-3-(hexadecanoyloxy)propoxy)phosphinic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@](O)(CO)COP(O)(=O)OC[C@@]([H])(COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCCCCC\C=C/CCCCCC |
|---|
| InChI Identifier | InChI=1S/C38H73O10P/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-37(41)45-33-36(34-47-49(43,44)46-32-35(40)31-39)48-38(42)30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h14,16,35-36,39-40H,3-13,15,17-34H2,1-2H3,(H,43,44)/b16-14-/t35-,36+/m0/s1 |
|---|
| InChI Key | NOFOTAHTYLXZNV-DCQLZOMZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphatidylglycerols. These are glycerophosphoglycerols in which two fatty acids are bonded to the 1-glycerol moiety through ester linkages. As is the case with diacylglycerols, phosphatidylglycerols can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoglycerols |
|---|
| Direct Parent | Phosphatidylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2-diacylglycerophosphoglycerol
- Fatty acid ester
- Dialkyl phosphate
- Dicarboxylic acid or derivatives
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- 1,2-diol
- Carboxylic acid ester
- Secondary alcohol
- Carboxylic acid derivative
- Organic oxide
- Organooxygen compound
- Alcohol
- Organic oxygen compound
- Primary alcohol
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Intracellular membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gki-5290821800-3c3c5f3b0ece868a4cf8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00p1-6391412200-74b513bb205005144338 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0bvj-9163221000-4f1fc5305749f6996295 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ap0-0190401300-13fffa7cf14e435a56ee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a70-5291100000-f93fbaff53be8341b2f4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9020000000-45ba6877001c9f7c4f7d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000000900-dbc822615d212739308e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-10k9-0091300400-7b0bf000719096af4470 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-10k9-0191300400-faaabd9809053c30be52 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|