| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-07-17 17:36:38 UTC |
|---|
| Update Date | 2020-05-21 16:27:32 UTC |
|---|
| BMDB ID | BMDB0062411 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
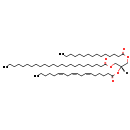
| Common Name | TG(15:0/18:3(6Z,9Z,12Z)/24:0) |
|---|
| Description | TG(15:0/18:3(6Z,9Z,12Z)/24:0), also known as tag(15:0/18:3/24:0) or tag(57:3), belongs to the class of organic compounds known as triacylglycerols. These are glycerides consisting of three fatty acid chains covalently bonded to a glycerol molecule through ester linkages. TG(15:0/18:3(6Z,9Z,12Z)/24:0) is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). TG(15:0/18:3(6Z,9Z,12Z)/24:0) can be biosynthesized from DG(15:0/18:3(6Z,9Z,12Z)/0:0) and tetracosanoyl-CoA; which is mediated by the enzyme diacylglycerol O-acyltransferase. In cattle, TG(15:0/18:3(6Z,9Z,12Z)/24:0) is involved in the metabolic pathway called de novo triacylglycerol biosynthesis TG(15:0/18:3(6Z,9Z,12Z)/24:0) pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Pentadecanoyl-2-(6Z,9Z,12Z-octadecatrienoyl)-3-tetracosanoyl-glycerol | HMDB | | 1-Pentadecanoyl-2-g-linolenoyl-3-lignoceroyl-glycerol | HMDB | | TAG(15:0/18:3/24:0) | HMDB | | TAG(57:3) | HMDB | | TG(15:0/18:3/24:0) | HMDB | | TG(57:3) | HMDB | | Tracylglycerol(15:0/18:3/24:0) | HMDB | | Tracylglycerol(57:3) | HMDB | | Triacylglycerol | HMDB | | Triglyceride | HMDB | | TG(15:0/18:3(6Z,9Z,12Z)/24:0) | Lipid Annotator |
|
|---|
| Chemical Formula | C60H110O6 |
|---|
| Average Molecular Weight | 927.534 |
|---|
| Monoisotopic Molecular Weight | 926.830241262 |
|---|
| IUPAC Name | (2S)-2-[(6Z,9Z,12Z)-octadeca-6,9,12-trienoyloxy]-3-(pentadecanoyloxy)propyl tetracosanoate |
|---|
| Traditional Name | (2S)-2-[(6Z,9Z,12Z)-octadeca-6,9,12-trienoyloxy]-3-(pentadecanoyloxy)propyl tetracosanoate |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@](COC(=O)CCCCCCCCCCCCCC)(COC(=O)CCCCCCCCCCCCCCCCCCCCCCC)OC(=O)CCCC\C=C/C\C=C/C\C=C/CCCCC |
|---|
| InChI Identifier | InChI=1S/C60H110O6/c1-4-7-10-13-16-19-22-25-27-28-29-30-31-32-34-35-38-41-44-47-50-53-59(62)65-56-57(55-64-58(61)52-49-46-43-40-37-24-21-18-15-12-9-6-3)66-60(63)54-51-48-45-42-39-36-33-26-23-20-17-14-11-8-5-2/h17,20,26,33,39,42,57H,4-16,18-19,21-25,27-32,34-38,40-41,43-56H2,1-3H3/b20-17-,33-26-,42-39-/t57-/m0/s1 |
|---|
| InChI Key | JAVPHHAAEYIKMM-KNGAKILKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triacylglycerols. These are glycerides consisting of three fatty acid chains covalently bonded to a glycerol molecule through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerolipids |
|---|
| Sub Class | Triradylcglycerols |
|---|
| Direct Parent | Triacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triacyl-sn-glycerol
- Tricarboxylic acid or derivatives
- Fatty acid ester
- Fatty acyl
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 21.84 | Extrapolated |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000000009-c80e1b1d5299399421e6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0000000009-c80e1b1d5299399421e6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a7b-0000049003-b1677da852d229414435 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01dm-0096002001-6f09daf0bb420dae91f9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01bd-0098000000-a771f8c86e8419cee39c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05tg-3195000000-cb59c1d4f028c66bee38 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0064016009-152f1f6c911743d1f9ee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ar3-0098101000-2fd857653857400277a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05ox-2096000000-02781476bc00c7944a5e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000000009-499958d627be3f47b75c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0000000009-499958d627be3f47b75c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a7b-0010049003-21fa190de0e2ca72b650 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-2020012059-5e8abe134b19b8beb316 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0btc-9021001081-296d1b4cda466b94ffea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ikc-1694110050-f734473c5a0cc3790460 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000000009-b87e5e35509ff93b441d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0000000009-b87e5e35509ff93b441d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0000000009-b87e5e35509ff93b441d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000000009-25ce470103f7b6b5774e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0000000009-25ce470103f7b6b5774e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ujo-0004009004-60caec21e58d9872d6c4 | View in MoNA |
|---|
|
|---|