| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2018-10-03 16:32:39 UTC |
|---|
| Update Date | 2020-06-04 20:47:23 UTC |
|---|
| BMDB ID | BMDB0063603 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | atraton |
|---|
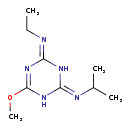
| Description | atraton belongs to the class of organic compounds known as 1,3,5-triazine-2,4-diamines. These are aromatic compounds containing a 1,3,5-triazine ring which is 2,4-disusbtituted wit amine groups. Based on a literature review a significant number of articles have been published on atraton. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Methoxy-4-ethylamino-6-isopropylamino-S-triazine | ChEBI | | 2-Methoxy-4-isopropylamino-6-ethylamino-S-triazine | ChEBI | | Atratone | MeSH | | N-Ethyl-6-methoxy-n'-(1-methylethyl)-1,3,5-triazine-2,4-diamine | MeSH | | NEMMTD CPD | MeSH |
|
|---|
| Chemical Formula | C9H17N5O |
|---|
| Average Molecular Weight | 211.2642 |
|---|
| Monoisotopic Molecular Weight | 211.143310191 |
|---|
| IUPAC Name | N-[4-(ethylimino)-6-methoxy-1,2,3,4-tetrahydro-1,3,5-triazin-2-ylidene]propan-2-amine |
|---|
| Traditional Name | N-[4-(ethylimino)-6-methoxy-1,3-dihydro-1,3,5-triazin-2-ylidene]propan-2-amine |
|---|
| CAS Registry Number | 1610-17-9 |
|---|
| SMILES | CCN=C1NC(NC(OC)=N1)=NC(C)C |
|---|
| InChI Identifier | InChI=1S/C9H17N5O/c1-5-10-7-12-8(11-6(2)3)14-9(13-7)15-4/h6H,5H2,1-4H3,(H2,10,11,12,13,14) |
|---|
| InChI Key | PXWUKZGIHQRDHL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3,5-triazine-2,4-diamines. These are aromatic compounds containing a 1,3,5-triazine ring which is 2,4-disusbtituted wit amine groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Triazines |
|---|
| Sub Class | Aminotriazines |
|---|
| Direct Parent | 1,3,5-triazine-2,4-diamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2,4-diamine-s-triazine
- 2-methoxy-1,3,5-triazine
- Alkoxy-s-triazine
- Alkyl aryl ether
- Secondary aliphatic/aromatic amine
- N-aliphatic s-triazine
- 1,3,5-triazine
- Heteroaromatic compound
- Ether
- Secondary amine
- Azacycle
- Organic nitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00kg-5900000000-216b7e78c1aba68ff5f4 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0ldi-9200000000-9db63dbbba7bd43e0f55 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-0uxr-9500000000-ee9595d02129d1fd5330 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-00di-6900000000-77cc1a8317da7485c8c5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-00di-1930000000-0c5b9b4d356186635508 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-03di-0090000000-3ddcb54164a37b28b77f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-03di-0290000000-63af51ef1c93d0b81775 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0gb9-9600000000-b761f36a2db4422e189c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-0g4i-6900000000-0caca22f3b93fa758ce1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-00di-0900000000-f918fcff5d56ed586c89 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-00di-0900000000-77603558036562b2be8f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-00di-2910000000-1103e74f7d75bf7a75b5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-00di-2910000000-89ab7a0c89a7bf4dc4f7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-03k9-0790000000-b60588501817acfc3b88 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-03k9-0890000000-bc11463e2d3fd929ef94 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-03di-0090000000-02d508284bce569095a6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-03di-0090000000-d133f146ab526bcf22de | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-03di-0090000000-d21c58c4d4136d906d1e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-0g6r-6900000000-66c7b9b6c71b6bbc164e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0gb9-9600000000-63c41836bf1d60e3d06b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0ldi-9200000000-c7d621ee01645d3ec4a6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ik9-2930000000-54871d12865e265a81bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a5c-9510000000-efa0eb53dadbaddfa137 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9500000000-ab14bdb254528c10fd51 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-d56890c8d2173c112a20 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9110000000-1c61534d1df00f7da2ef | View in MoNA |
|---|
|
|---|