| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-02-10 19:45:57 UTC |
|---|
| Update Date | 2020-05-11 22:39:41 UTC |
|---|
| BMDB ID | BMDB0063680 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | (-)-Dihydrocarveol |
|---|
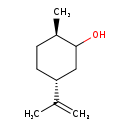
| Description | (-)-Dihydrocarveol belongs to the class of organic compounds known as menthane monoterpenoids. These are monoterpenoids with a structure based on the o-, m-, or p-menthane backbone. P-menthane consists of the cyclohexane ring with a methyl group and a (2-methyl)-propyl group at the 1 and 4 ring position, respectively. The o- and m- menthanes are much rarer, and presumably arise by alkyl migration of p-menthanes. Based on a literature review a small amount of articles have been published on (-)-Dihydrocarveol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1R,2R,4R)-Dihydrocarveol | HMDB | | (1R,2R,4R)-P-Menth-8-en-2-ol | HMDB | | (1R,2R,5R)-2-Methyl-5-(prop-1-en-2-yl)cyclohexanol | HMDB | | (1R,2R,5R)-5-Isopropenyl-2-methylcyclohexanol | HMDB |
|
|---|
| Chemical Formula | C10H18O |
|---|
| Average Molecular Weight | 154.2493 |
|---|
| Monoisotopic Molecular Weight | 154.135765198 |
|---|
| IUPAC Name | (2R,5R)-2-methyl-5-(prop-1-en-2-yl)cyclohexan-1-ol |
|---|
| Traditional Name | (2R,5R)-2-methyl-5-(prop-1-en-2-yl)cyclohexan-1-ol |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | C[C@@H]1CC[C@H](CC1O)C(C)=C |
|---|
| InChI Identifier | InChI=1S/C10H18O/c1-7(2)9-5-4-8(3)10(11)6-9/h8-11H,1,4-6H2,2-3H3/t8-,9-,10?/m1/s1 |
|---|
| InChI Key | KRCZYMFUWVJCLI-MGRQHWMJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as menthane monoterpenoids. These are monoterpenoids with a structure based on the o-, m-, or p-menthane backbone. P-menthane consists of the cyclohexane ring with a methyl group and a (2-methyl)-propyl group at the 1 and 4 ring position, respectively. The o- and m- menthanes are much rarer, and presumably arise by alkyl migration of p-menthanes. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Menthane monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-menthane monoterpenoid
- Monocyclic monoterpenoid
- Cyclohexanol
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9200000000-93463c7f16a8815c2f6a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-06yx-9610000000-180a38e0cd6d5a98561b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-1900000000-426222b005de9ed263f7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-7900000000-92669f5cd45165c58a8a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0api-9100000000-a02a955b5b196ac361c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-a7c7b44c65ac0d9983a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0900000000-08b2ced2412775e44ff3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f79-8900000000-ef61141645310f0e0a80 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udr-0900000000-6cb010728dc534ba406c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0900000000-318736dc4101f232fc4c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-2900000000-9492df1cf75725527190 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0532-9400000000-959ea8fcd37ca9a711e5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00l5-9100000000-66dc6c3c3f3184c185af | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9000000000-09a8ec5e3424e50d627f | View in MoNA |
|---|
|
|---|