| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:01:45 UTC |
|---|
| Update Date | 2020-04-22 18:56:16 UTC |
|---|
| BMDB ID | BMDB0096134 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 6-Sulfatoxymelatonin |
|---|
| Description | 6-Sulfatoxymelatonin belongs to the class of organic compounds known as n-acetyl-2-arylethylamines. N-acetyl-2-arylethylamines are compounds containing an acetamide group that is N-linked to an arylethylamine. Based on a literature review very few articles have been published on 6-Sulfatoxymelatonin. |
|---|
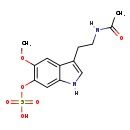
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-Sulphatoxymelatonin | Generator | | 6-Hydroxymelatonin sulfate ester | HMDB | | 6-Hydroxymelatoninsulfate | HMDB | | 6-Sulphatoxy melatonin | HMDB | | 6-Sulfatoxymelatonin, monosodium salt | HMDB | | N-{2-[5-methoxy-6-(sulfooxy)-1H-indol-3-yl]ethyl}ethanimidate | HMDB | | N-{2-[5-methoxy-6-(sulphooxy)-1H-indol-3-yl]ethyl}ethanimidate | HMDB | | N-{2-[5-methoxy-6-(sulphooxy)-1H-indol-3-yl]ethyl}ethanimidic acid | HMDB | | 6-Sulfatoxymelatonin | MeSH |
|
|---|
| Chemical Formula | C13H16N2O6S |
|---|
| Average Molecular Weight | 328.341 |
|---|
| Monoisotopic Molecular Weight | 328.072906944 |
|---|
| IUPAC Name | N-{2-[5-methoxy-6-(sulfooxy)-1H-indol-3-yl]ethyl}ethanimidic acid |
|---|
| Traditional Name | N-{2-[5-methoxy-6-(sulfooxy)-1H-indol-3-yl]ethyl}ethanimidic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]N(CCC1=CN([H])C2=CC(OS(O)(=O)=O)=C(OC)C=C12)C(C)=O |
|---|
| InChI Identifier | InChI=1S/C13H16N2O6S/c1-8(16)14-4-3-9-7-15-11-6-13(21-22(17,18)19)12(20-2)5-10(9)11/h5-7,15H,3-4H2,1-2H3,(H,14,16)(H,17,18,19) |
|---|
| InChI Key | QQEILXDLZRLTME-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acetyl-2-arylethylamines. N-acetyl-2-arylethylamines are compounds containing an acetamide group that is N-linked to an arylethylamine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Carboxylic acid derivatives |
|---|
| Direct Parent | N-acetyl-2-arylethylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acetyl-2-arylethylamine
- Arylsulfate
- 3-alkylindole
- Indole
- Indole or derivatives
- Anisole
- Alkyl aryl ether
- Substituted pyrrole
- Sulfuric acid monoester
- Sulfate-ester
- Sulfuric acid ester
- Benzenoid
- Organic sulfuric acid or derivatives
- Pyrrole
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Ether
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-5292000000-f1ca77e6283e10248e70 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00y0-4239000000-ce9669ca7ba45ea80a43 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002r-0096000000-644185cd9e83fce098a0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0670-0190000000-b0749fc1b0033ecd7068 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-2960000000-c22a03a1348cae4d0047 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0039000000-6bc2269d3bf379922180 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pds-2091000000-cb36d89600732ff22990 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-9020000000-e7b126fae9236afca71f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0292000000-afae50426af3edeed8c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0391000000-adb49bb2b3c3a28f904e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-0910000000-f1d0d98af3df3ee912c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0019000000-0dde34d32b6700465807 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052f-9010000000-2243b851e638e169a124 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0005-9310000000-90237867775ed7f0181f | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|