| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:02:14 UTC |
|---|
| Update Date | 2020-04-22 18:56:27 UTC |
|---|
| BMDB ID | BMDB0096163 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | xanthurenic acid 8-O-sulfate |
|---|
| Description | xanthurenic acid 8-O-sulfate belongs to the class of organic compounds known as quinoline carboxylic acids. These are quinolines in which the quinoline ring system is substituted by a carboxyl group at one or more positions. xanthurenic acid 8-O-sulfate is an extremely weak basic (essentially neutral) compound (based on its pKa). These are compounds containing a quinoline moiety bearing an hydroxyl group. |
|---|
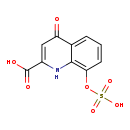
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Xanthurenate 8-O-sulfate | Generator | | Xanthurenate 8-O-sulphate | Generator | | Xanthurenic acid 8-O-sulfuric acid | Generator | | Xanthurenic acid 8-O-sulphuric acid | Generator | | 4-Hydroxy-8-(sulfooxy)quinoline-2-carboxylate | Generator | | 4-Hydroxy-8-(sulphooxy)quinoline-2-carboxylate | Generator | | 4-Hydroxy-8-(sulphooxy)quinoline-2-carboxylic acid | Generator | | Xanthurenic acid 8-O-sulfate | MeSH |
|
|---|
| Chemical Formula | C10H7NO7S |
|---|
| Average Molecular Weight | 285.23 |
|---|
| Monoisotopic Molecular Weight | 284.994322273 |
|---|
| IUPAC Name | 4-oxo-8-(sulfooxy)-1,4-dihydroquinoline-2-carboxylic acid |
|---|
| Traditional Name | 4-oxo-8-(sulfooxy)-1H-quinoline-2-carboxylic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OC(=O)C1=CC(=O)C2=CC=CC(OS(O)(=O)=O)=C2N1 |
|---|
| InChI Identifier | InChI=1S/C10H7NO7S/c12-7-4-6(10(13)14)11-9-5(7)2-1-3-8(9)18-19(15,16)17/h1-4H,(H,11,12)(H,13,14)(H,15,16,17) |
|---|
| InChI Key | FNGKEPQCRRMUOC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as quinoline carboxylic acids. These are quinolines in which the quinoline ring system is substituted by a carboxyl group at one or more positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Quinoline carboxylic acids |
|---|
| Direct Parent | Quinoline carboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Quinoline-2-carboxylic acid
- Dihydroquinolone
- Dihydroquinoline
- Arylsulfate
- Pyridine carboxylic acid
- Pyridine carboxylic acid or derivatives
- Pyridine
- Sulfuric acid monoester
- Sulfate-ester
- Benzenoid
- Sulfuric acid ester
- Organic sulfuric acid or derivatives
- Heteroaromatic compound
- Vinylogous amide
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Azacycle
- Carboxylic acid
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05s0-0590000000-8060cf73f3d668b9a07b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00xr-6091000000-44728b544b723b6aedb2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0090000000-f01e9c412da223661a2a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-0390000000-2645d9e916fd00bb63c2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00s9-2950000000-42f2ed6776bf900ef91f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-bf1bbb24dc616a64913e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uei-0490000000-361cab350a487498f26f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bu9-3910000000-1303a4423bc52dd8b0c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-3a0aa5fdda83c8a9b219 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0910000000-af7704fb758806a72d43 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0920000000-fb4ac1f6d26d35f443cc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-8d494c133d0d227d0b7e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-c62be4673f624406b78c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-3920000000-6e831a7b127d9df073e9 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|