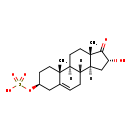
| 16a-Hydroxydehydroepiandrosterone 3-sulfate | Generator |
| 16a-Hydroxydehydroepiandrosterone 3-sulfuric acid | Generator |
| 16a-Hydroxydehydroepiandrosterone 3-sulphate | Generator |
| 16a-Hydroxydehydroepiandrosterone 3-sulphuric acid | Generator |
| 16alpha-Hydroxydehydroepiandrosterone 3-sulfuric acid | Generator |
| 16alpha-Hydroxydehydroepiandrosterone 3-sulphate | Generator |
| 16alpha-Hydroxydehydroepiandrosterone 3-sulphuric acid | Generator |
| 16α-Hydroxydehydroepiandrosterone 3-sulfate | Generator |
| 16α-hydroxydehydroepiandrosterone 3-sulfuric acid | Generator |
| 16α-Hydroxydehydroepiandrosterone 3-sulphate | Generator |
| 16α-hydroxydehydroepiandrosterone 3-sulphuric acid | Generator |
| (3b)-16a-Hydroxy-17-oxoandrost-5-en-3-yl sulfate | HMDB, Generator |
| (3b)-16a-Hydroxy-17-oxoandrost-5-en-3-yl sulfuric acid | HMDB, Generator |
| (3b)-16a-Hydroxy-17-oxoandrost-5-en-3-yl sulphate | HMDB, Generator |
| (3b)-16a-Hydroxy-17-oxoandrost-5-en-3-yl sulphuric acid | HMDB, Generator |
| (3b,16a)-16-Hydroxy-17-oxoandrost-5-en-3-yl sulfate | HMDB, Generator |
| (3b,16a)-16-Hydroxy-17-oxoandrost-5-en-3-yl sulfuric acid | HMDB, Generator |
| (3b,16a)-16-Hydroxy-17-oxoandrost-5-en-3-yl sulphate | HMDB, Generator |
| (3b,16a)-16-Hydroxy-17-oxoandrost-5-en-3-yl sulphuric acid | HMDB, Generator |
| (3beta)-16alpha-Hydroxy-17-oxoandrost-5-en-3-yl sulfate | HMDB, ChEBI |
| (3beta)-16alpha-Hydroxy-17-oxoandrost-5-en-3-yl sulfuric acid | HMDB, Generator |
| (3beta)-16alpha-Hydroxy-17-oxoandrost-5-en-3-yl sulphate | HMDB, Generator |
| (3beta)-16alpha-Hydroxy-17-oxoandrost-5-en-3-yl sulphuric acid | HMDB, Generator |
| (3beta,16alpha)-16-Hydroxy-17-oxoandrost-5-en-3-yl sulfate | HMDB, ChEBI |
| (3beta,16alpha)-16-Hydroxy-17-oxoandrost-5-en-3-yl sulfuric acid | HMDB, Generator |
| (3beta,16alpha)-16-Hydroxy-17-oxoandrost-5-en-3-yl sulphate | HMDB, Generator |
| (3beta,16alpha)-16-Hydroxy-17-oxoandrost-5-en-3-yl sulphuric acid | HMDB, Generator |
| (3β)-16α-Hydroxy-17-oxoandrost-5-en-3-yl sulfate | HMDB, Generator |
| (3β)-16α-hydroxy-17-oxoandrost-5-en-3-yl sulfuric acid | HMDB, Generator |
| (3β)-16α-Hydroxy-17-oxoandrost-5-en-3-yl sulphate | HMDB, Generator |
| (3β)-16α-hydroxy-17-oxoandrost-5-en-3-yl sulphuric acid | HMDB, Generator |
| (3β,16α)-16-Hydroxy-17-oxoandrost-5-en-3-yl sulfate | HMDB, Generator |
| (3β,16α)-16-hydroxy-17-oxoandrost-5-en-3-yl sulfuric acid | HMDB, Generator |
| (3β,16α)-16-Hydroxy-17-oxoandrost-5-en-3-yl sulphate | HMDB, Generator |
| (3β,16α)-16-hydroxy-17-oxoandrost-5-en-3-yl sulphuric acid | HMDB, Generator |
| 16a-Hydroxy-DHEA 3-sulfate | HMDB, Generator |
| 16a-Hydroxy-DHEA 3-sulfuric acid | HMDB, Generator |
| 16a-Hydroxy-DHEA 3-sulphate | HMDB, Generator |
| 16a-Hydroxy-DHEA 3-sulphuric acid | HMDB, Generator |
| 16alpha-Hydroxy-DHEA 3-sulfate | HMDB, ChEBI |
| 16alpha-Hydroxy-DHEA 3-sulfuric acid | HMDB, Generator |
| 16alpha-Hydroxy-DHEA 3-sulphate | HMDB, Generator |
| 16alpha-Hydroxy-DHEA 3-sulphuric acid | HMDB, Generator |
| 16α-Hydroxy-DHEA 3-sulfate | HMDB, Generator |
| 16α-hydroxy-DHEA 3-sulfuric acid | HMDB, Generator |
| 16α-Hydroxy-DHEA 3-sulphate | HMDB, Generator |
| 16α-hydroxy-DHEA 3-sulphuric acid | HMDB, Generator |
| 3b-Sulfooxy-16a-hydroxyandrost-5-en-17-one | HMDB, Generator |
| 3b-Sulphooxy-16a-hydroxyandrost-5-en-17-one | HMDB, Generator |
| 3beta-Sulfooxy-16alpha-hydroxyandrost-5-en-17-one | HMDB, ChEBI |
| 3beta-Sulphooxy-16alpha-hydroxyandrost-5-en-17-one | HMDB, Generator |
| 3β-Sulfooxy-16α-hydroxyandrost-5-en-17-one | HMDB, Generator |
| 3β-sulphooxy-16α-hydroxyandrost-5-en-17-one | HMDB, Generator |
| (3beta,16alpha)-16-Hydroxy-3-(sulfooxy)androst-5-en-17-one | HMDB |
| (3β,16α)-16-Hydroxy-3-(sulfooxy)androst-5-en-17-one | HMDB |
| 16alpha-Hydroxy DHEA 3-sulfate | HMDB |
| 16alpha-Hydroxy DHEA 3-sulphate | HMDB |
| 16alpha-Hydroxy DHEA sulfate | HMDB |
| 16alpha-Hydroxy DHEA sulphate | HMDB |
| 16alpha-Hydroxy-DHEA sulfate | HMDB |
| 16alpha-Hydroxy-DHEA sulphate | HMDB |
| 16alpha-Hydroxydehydroepiandrosterone 3-sulfate | HMDB |
| 16alpha-Hydroxydehydroisoandrosterone sulfate | HMDB |
| 16alpha-Hydroxydehydroisoandrosterone sulphate | HMDB |
| 16alpha-OH DHEAS | HMDB |
| 16alpha-OH-DHEA-3S | HMDB |
| 16alpha-OH-DHEA-S | HMDB |
| 16alpha-OH-DHEAS | HMDB |
| 16α-Hydroxy DHEA 3-sulfate | HMDB |
| 16α-Hydroxy DHEA 3-sulphate | HMDB |
| 16α-Hydroxy DHEA sulfate | HMDB |
| 16α-Hydroxy DHEA sulphate | HMDB |
| 16α-Hydroxy-DHEA sulfate | HMDB |
| 16α-Hydroxy-DHEA sulphate | HMDB |
| 16α-Hydroxydehydroisoandrosterone sulfate | HMDB |
| 16α-Hydroxydehydroisoandrosterone sulphate | HMDB |
| 16α-OH DHEAS | HMDB |
| 16α-OH-DHEA-3S | HMDB |
| 16α-OH-DHEA-S | HMDB |
| 16α-OH-DHEAS | HMDB |
| 3beta,16alpha-Dihydroxy-5-androsten-17-one 3-sulfate | HMDB |
| 3beta,16alpha-Dihydroxy-5-androsten-17-one 3-sulphate | HMDB |
| 3β,16α-Dihydroxy-5-androsten-17-one 3-sulfate | HMDB |
| 3β,16α-Dihydroxy-5-androsten-17-one 3-sulphate | HMDB |
| (3beta,16alpha)-3,16-Dihydroxyandrost-5-en-17-one sulfate | HMDB |
| (3beta,16alpha)-3,16-Dihydroxyandrost-5-en-17-one sulphate | HMDB |
| (3β,16α)-3,16-Dihydroxyandrost-5-en-17-one sulfate | HMDB |
| (3β,16α)-3,16-Dihydroxyandrost-5-en-17-one sulphate | HMDB |
| 3b,16a-Dihydroxyandrostenone sulfate | HMDB |
| 3b,16a-Dihydroxyandrostenone sulphate | HMDB |
| 3beta,16alpha-Dihydroxyandrost-5-en-17-one sulfate | HMDB |
| 3beta,16alpha-Dihydroxyandrost-5-en-17-one sulphate | HMDB |
| 3beta,16alpha-Dihydroxyandrostenone sulfate | HMDB |
| 3beta,16alpha-Dihydroxyandrostenone sulphate | HMDB |
| 3β,16α-Dihydroxyandrost-5-en-17-one sulfate | HMDB |
| 3β,16α-Dihydroxyandrost-5-en-17-one sulphate | HMDB |
| 3β,16α-Dihydroxyandrostenone sulfate | HMDB |
| 3β,16α-Dihydroxyandrostenone sulphate | HMDB |