| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:03:51 UTC |
|---|
| Update Date | 2020-03-13 22:44:06 UTC |
|---|
| BMDB ID | BMDB0096261 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
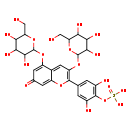
| Common Name | {2,6-dihydroxy-4-[7-oxo-3,5-bis({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy})-7H-chromen-2-yl]phenoxy}dihydroxyoxo-λ⁶-sulfanylium |
|---|
| Description | {2,6-dihydroxy-4-[7-oxo-3,5-bis({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy})-7H-chromen-2-yl]phenoxy}dihydroxyoxo-λ⁶-sulfanylium belongs to the class of organic compounds known as flavonoid-3-o-glycosides. These are phenolic compounds containing a flavonoid moiety which is O-glycosidically linked to carbohydrate moiety at the C3-position. Based on a literature review very few articles have been published on {2,6-dihydroxy-4-[7-oxo-3,5-bis({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy})-7H-chromen-2-yl]phenoxy}dihydroxyoxo-λ⁶-sulfanylium. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| {2,6-dihydroxy-4-[7-oxo-3,5-bis({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy})-7H-chromen-2-yl]phenoxy}dihydroxyoxo-λ⁶-sulphanylium | Generator | | {[6-({2-[3,5-dihydroxy-4-(sulphooxy)phenyl]-7-oxo-3-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-7H-chromen-5-yl}oxy)-3,4,5-trihydroxyoxan-2-yl]methyl}oxidanium | Generator, HMDB |
|
|---|
| Chemical Formula | C27H31O20S |
|---|
| Average Molecular Weight | 707.58 |
|---|
| Monoisotopic Molecular Weight | 707.112390992 |
|---|
| IUPAC Name | {2,6-dihydroxy-4-[7-oxo-3,5-bis({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy})-7H-chromen-2-yl]phenoxy}dihydroxyoxo-lambda6-sulfanylium |
|---|
| Traditional Name | 2,6-dihydroxy-4-[7-oxo-3,5-bis({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy})chromen-2-yl]phenoxydihydroxyoxo-lambda6-sulfanylium |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | OCC1OC(OC2=CC(=O)C=C3OC(=C(OC4OC(CO)C(O)C(O)C4O)C=C23)C2=CC(O)=C(O[S+](O)(O)=O)C(O)=C2)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C27H30O20S/c28-6-16-18(33)20(35)22(37)26(45-16)43-14-4-9(30)3-13-10(14)5-15(44-27-23(38)21(36)19(34)17(7-29)46-27)24(42-13)8-1-11(31)25(12(32)2-8)47-48(39,40)41/h1-5,16-23,26-29,33-38H,6-7H2,(H3-,31,32,39,40,41)/p+1 |
|---|
| InChI Key | DRABZUZWNLTDHV-UHFFFAOYSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as flavonoid-3-o-glycosides. These are phenolic compounds containing a flavonoid moiety which is O-glycosidically linked to carbohydrate moiety at the C3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavonoid glycosides |
|---|
| Direct Parent | Flavonoid-3-O-glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Flavonoid-3-o-glycoside
- 3'-hydroxyflavonoid
- Hydroxyflavonoid
- Monohydroxyflavonoid
- Phenolic glycoside
- Glycosyl compound
- O-glycosyl compound
- Benzopyran
- 1-benzopyran
- Phenoxy compound
- Resorcinol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Oxane
- Monosaccharide
- Benzenoid
- Heteroaromatic compound
- Vinylogous ester
- Cyclic ketone
- Secondary alcohol
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Polyol
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Primary alcohol
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_15) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0002091000-dcf3cf401b56a33a8a47 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-003r-0109280000-6c554f5ad50f64da441e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-1219120000-0dcea9789b8ab87af7b3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0101093100-62a508211596d7183678 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000t-0104494000-9e5b3a99476c25e7cf9d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002k-8802390000-5e71ed3ea9ed3598d7a2 | View in MoNA |
|---|
|
|---|