| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-10 17:03:53 UTC |
|---|
| Update Date | 2020-04-22 18:57:01 UTC |
|---|
| BMDB ID | BMDB0096263 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3-(3,5-dihydroxyphenyl)-4-(2-{3-[6-hydroxy-3-(3-hydroxyphenyl)-2-phenyl-2,3-dihydro-1-benzofuran-4-yl]-2-phenyl-2,3-dihydro-1-benzofuran-5-yl}ethenyl)-2-(4-hydroxyphenyl)-2,3-dihydro-1-benzofuran-5,6-diol |
|---|
| Description | 3-(3,5-dihydroxyphenyl)-4-(2-{3-[6-hydroxy-3-(3-hydroxyphenyl)-2-phenyl-2,3-dihydro-1-benzofuran-4-yl]-2-phenyl-2,3-dihydro-1-benzofuran-5-yl}ethenyl)-2-(4-hydroxyphenyl)-2,3-dihydro-1-benzofuran-5,6-diol belongs to the class of organic compounds known as 2-arylbenzofuran flavonoids. These are phenylpropanoids containing the 2-phenylbenzofuran moiety. Based on a literature review very few articles have been published on 3-(3,5-dihydroxyphenyl)-4-(2-{3-[6-hydroxy-3-(3-hydroxyphenyl)-2-phenyl-2,3-dihydro-1-benzofuran-4-yl]-2-phenyl-2,3-dihydro-1-benzofuran-5-yl}ethenyl)-2-(4-hydroxyphenyl)-2,3-dihydro-1-benzofuran-5,6-diol. |
|---|
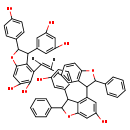
| Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C56H42O10 |
|---|
| Average Molecular Weight | 874.942 |
|---|
| Monoisotopic Molecular Weight | 874.277797552 |
|---|
| IUPAC Name | 3-(3,5-dihydroxyphenyl)-4-(2-{3-[6-hydroxy-3-(3-hydroxyphenyl)-2-phenyl-2,3-dihydro-1-benzofuran-4-yl]-2-phenyl-2,3-dihydro-1-benzofuran-5-yl}ethenyl)-2-(4-hydroxyphenyl)-2,3-dihydro-1-benzofuran-5,6-diol |
|---|
| Traditional Name | 3-(3,5-dihydroxyphenyl)-4-(2-{3-[6-hydroxy-3-(3-hydroxyphenyl)-2-phenyl-2,3-dihydro-1-benzofuran-4-yl]-2-phenyl-2,3-dihydro-1-benzofuran-5-yl}ethenyl)-2-(4-hydroxyphenyl)-2,3-dihydro-1-benzofuran-5,6-diol |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H]C(=C([H])C1=C2C(C(OC2=CC(O)=C1O)C1=CC=C(O)C=C1)C1=CC(O)=CC(O)=C1)C1=CC2=C(OC(C2C2=C3C(C(OC3=CC(O)=C2)C2=CC=CC=C2)C2=CC(O)=CC=C2)C2=CC=CC=C2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C56H42O10/c57-36-18-16-33(17-19-36)55-49(35-24-38(59)26-39(60)25-35)51-41(53(63)44(62)29-47(51)66-55)20-14-30-15-21-45-42(22-30)50(56(64-45)32-10-5-2-6-11-32)43-27-40(61)28-46-52(43)48(34-12-7-13-37(58)23-34)54(65-46)31-8-3-1-4-9-31/h1-29,48-50,54-63H/b20-14- |
|---|
| InChI Key | AQVZAGJIDKQXLL-ZHZULCJRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2-arylbenzofuran flavonoids. These are phenylpropanoids containing the 2-phenylbenzofuran moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | 2-arylbenzofuran flavonoids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | 2-arylbenzofuran flavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-arylbenzofuran flavonoid
- Neolignan skeleton
- 1-phenylcoumaran
- Stilbene
- Benzofuran
- Coumaran
- Resorcinol
- Styrene
- Alkyl aryl ether
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Benzenoid
- Monocyclic benzene moiety
- Ether
- Organoheterocyclic compound
- Oxacycle
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0003130390-a0e78ea3bc7cf70e5dce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bta-0719162360-75ad24bf1361d3c14a14 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0adi-1950222510-758c95525d8cc8696cf8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000000090-8ba7b364ed09ffaadf2d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-1001010190-527bf0ed371742be4a18 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ov-7010000790-f69da66d5405a2743adf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000000090-4ecf91868c71cb55a2dd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0000001190-5126052a2f2f181f4889 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01dm-4310104590-0a2eb0070348a8d55e97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000000190-eeff5db8a24c995d1f61 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1000100290-8beae8c54a551d72b3de | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ou-9600006770-d8cac2db7c45297a1b45 | View in MoNA |
|---|
|
|---|