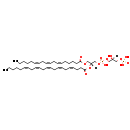| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2020-03-25 16:13:25 UTC |
|---|
| Update Date | 2020-05-11 19:22:43 UTC |
|---|
| BMDB ID | BMDB0096795 |
|---|
| Secondary Accession Numbers | None |
|---|
| Metabolite Identification |
|---|
| Common Name | PGP(18:3(6Z,9Z,12Z)/22:5(4Z,7Z,10Z,13Z,16Z)) |
|---|
| Description | PGP(18:3(6Z,9Z,12Z)/22:5(4Z,7Z,10Z,13Z,16Z)) belongs to the class of glycerophosphoglycerophosphates, also called phosphatidylglycerophosphates (PGPs). These lipids contain a common glycerophosphate skeleton linked to at least one fatty acyl chain and a glycero-3-phosphate moiety. As is the case with diacylglycerols, phosphatidylglycerophosphates can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PGP(18:3(6Z,9Z,12Z)/22:5(4Z,7Z,10Z,13Z,16Z)), in particular, consists of one 6Z,9Z,12Z-octadecatrienoyl chain to the C-1 atom, and one 4Z,7Z,10Z,13Z,16Z-docosapentaenoyl to the C-2 atom. In E. coli, PGPs can be found in the cytoplasmic membrane. The are synthesized by the addition of glycerol 3-phosphate to a CDP-diacylglycerol. In turn, PGPs are dephosphorylated to Phosphatidylglycerols (PGs) by the enzyme Phosphatidylglycerophosphatase. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-sn-phosphatidyl-1'-sn-glycerol 3'-phosphoric acid | Lipid Annotator, HMDB | | PGP(40:8) | Lipid Annotator, HMDB | | PGP(18:3/22:5) | Lipid Annotator, HMDB | | 1-g-linolenoyl-2-osbondoyl-sn-glycero-3-phospho-(1'-sn-glycerol-3'-phosphate) | Lipid Annotator, HMDB | | 1-(6Z,9Z,12Z-octadecatrienoyl)-2-(4Z,7Z,10Z,13Z,16Z-docosapentaenoyl)-sn-glycero-3-phospho-(1'-sn-glycerol-3'-phosphate) | Lipid Annotator, HMDB | | PGP(18:3(6Z,9Z,12Z)/22:5(4Z,7Z,10Z,13Z,16Z)) | Lipid Annotator | | 1-gamma-Linolenoyl-2-osbondoyl-sn-glycero-3-phospho-(1'-sn-glycerol-3'-phosphate) | HMDB | | PGP(18:3n6/22:5n6) | HMDB | | PGP(18:3W6/22:5W6) | HMDB | | [(2S)-3-({[(2R)-2-[(7Z,10Z,13Z,16Z)-docosa-4,7,10,13,16-pentaenoyloxy]-3-[(6Z,9Z,12Z)-octadeca-6,9,12-trienoyloxy]propoxy](hydroxy)phosphoryl}oxy)-2-hydroxypropoxy]phosphonate | Generator |
|
|---|
| Chemical Formula | C46H76O13P2 |
|---|
| Average Molecular Weight | 899.0354 |
|---|
| Monoisotopic Molecular Weight | 898.476115542 |
|---|
| IUPAC Name | [(2S)-3-({[(2R)-2-[(4Z,7Z,10Z,13Z,16Z)-docosa-4,7,10,13,16-pentaenoyloxy]-3-[(6Z,9Z,12Z)-octadeca-6,9,12-trienoyloxy]propoxy](hydroxy)phosphoryl}oxy)-2-hydroxypropoxy]phosphonic acid |
|---|
| Traditional Name | (2S)-3-{[(2R)-2-[(4Z,7Z,10Z,13Z,16Z)-docosa-4,7,10,13,16-pentaenoyloxy]-3-[(6Z,9Z,12Z)-octadeca-6,9,12-trienoyloxy]propoxy(hydroxy)phosphoryl]oxy}-2-hydroxypropoxyphosphonic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@](O)(COP(O)(O)=O)COP(O)(=O)OC[C@@]([H])(COC(=O)CCCC\C=C/C\C=C/C\C=C/CCCCC)OC(=O)CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCCC |
|---|
| InChI Identifier | InChI=1S/C46H76O13P2/c1-3-5-7-9-11-13-15-17-19-20-21-22-24-26-28-30-32-34-36-38-46(49)59-44(42-58-61(53,54)57-40-43(47)39-56-60(50,51)52)41-55-45(48)37-35-33-31-29-27-25-23-18-16-14-12-10-8-6-4-2/h11-14,17-19,21-23,26-29,32,34,43-44,47H,3-10,15-16,20,24-25,30-31,33,35-42H2,1-2H3,(H,53,54)(H2,50,51,52)/b13-11-,14-12-,19-17-,22-21-,23-18-,28-26-,29-27-,34-32-/t43-,44+/m0/s1 |
|---|
| InChI Key | HZYRGKIRVUKELD-MYMQDKSKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphatidylglycerophosphates. These are glycerophosphoglycerophosphates in which two fatty acids are bonded to the 1-glycerol moiety through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoglycerophosphates |
|---|
| Direct Parent | Phosphatidylglycerophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diacylglycerophosphoglycerophosphate
- Sn-glycerol-3-phosphate
- Fatty acid ester
- Monoalkyl phosphate
- Dialkyl phosphate
- Dicarboxylic acid or derivatives
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Secondary alcohol
- Carboxylic acid ester
- Carboxylic acid derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Intracellular membrane
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03ea-1396035280-94f209ab44db31c981b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-3495023320-afe1c783a828adc182b2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9377104500-e5c892b9cc1bbc8c42ce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-4092020120-8f2d246878a692c21530 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9050000000-2a50295d44f15fc2b0d0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-534a65ecdbf6ea27fa02 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-2000006090-9ab19cb4228f51d6fddf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pba-1200005940-853a83bc95572737a113 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01x0-1111971100-56e6ab3a6e814c3d2742 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000000090-28ccea0a1307488f812b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-9052014160-d04525be6851768c42eb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-8092000020-90428844d654e2aba56e | View in MoNA |
|---|
|
|---|